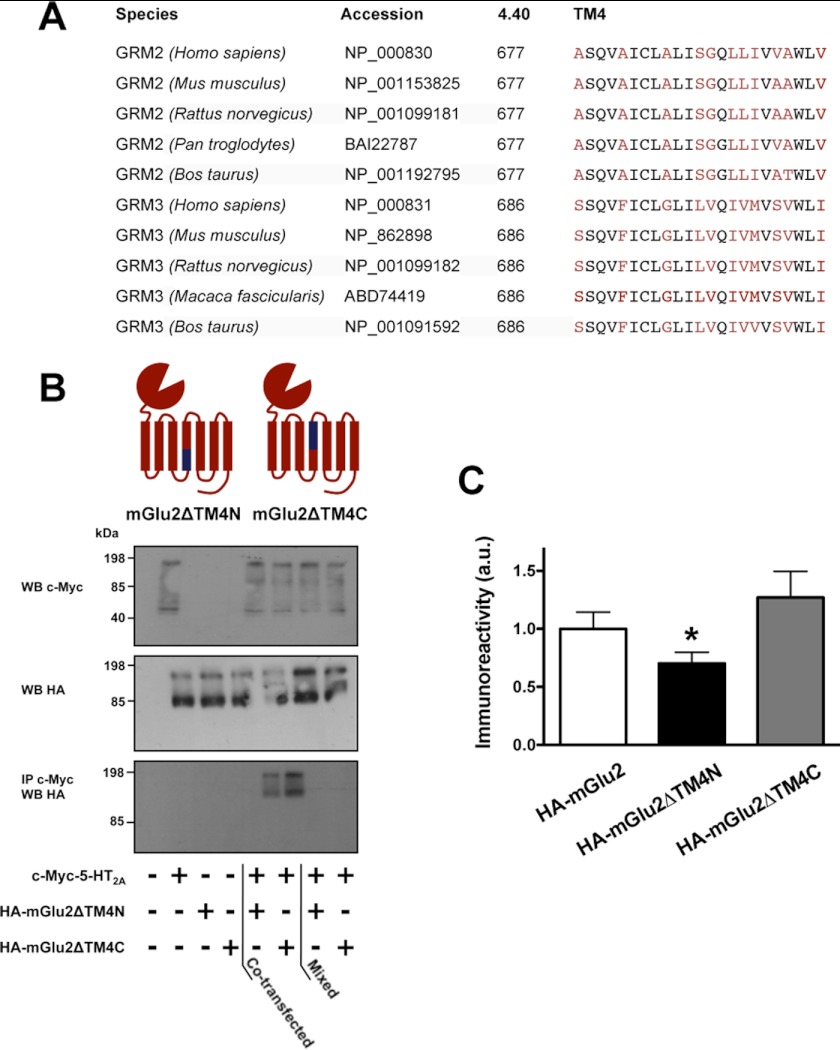
FIGURE 3.
Intracellular end of TM4 of mGlu2 mediates complex formation with the 5-HT2A receptor. A, multiple sequence alignment of the transmembrane domain 4 (TM4) of mGlu2 (GRM2) and mGlu3 (GRM3) receptors. All residues were identified by the Ballesteros-Weinstein numbering system (i.e. the most conserved residue in the TM in which they are located is assigned the position index “50”), as well as by the residue numbers of the amino acid sequences in the species shown. Residues that are different between mGlu2 and mGlu3 receptors are highlighted in red. B, co-immunoprecipitation (IP) experiments of c-Myc-5-HT2A and HA-mGlu2ΔTM4N or HA-mGlu2ΔTM4C in co-transfected HEK293 cells. The presence of the intracellular end of TM4, but not the extracellular end of TM4, of the mGlu2 receptor is necessary to co-immunoprecipitate with the 5-HT2A receptor. Schematic of mGlu2/mGlu3 chimeras studied are shown. For a control, cells separately expressing the c-Myc- or HA-tagged forms were mixed. Similar findings were obtained in two other independent experiments. WB, Western blot. C, co-immunoprecipitation experiments of c-Myc-5-HT2A and HA-mGlu2, HA-mGlu2ΔTM4N, or HA-mGlu2ΔTM4C in co-transfected HEK293 cells. Co-immunoprecipitation was decreased by substitution of residues Ala-6774.40, Ala-6814.44, and Ala-6854.48 in mGlu2 for Ser-6864.40, Phe-6904.44, and Gly-6944.48 in mGlu3 (HA-mGlu2ΔTM4N), but not by substitution of residues Ser-6884.51, Gly-6894.52, Leu-6914.54, Leu-6924.55, Ile-6934.56, Val-6954.58, Ala-6964.59, and Val-6994.62 in mGlu2 for Leu-6974.51, Val-6984.52, Ile-7004.54, Val-7014.55, Met-7024.56, Ser-7044.58, Val-7054.59, and Ile-7084.62 in mGlu3 (HA-mGlu2ΔTM4C), as compared with HA-mGlu2 (n = 9). *, p < 0.05; Bonferroni's post hoc test of one-way ANOVA. Error bars represent S.E.