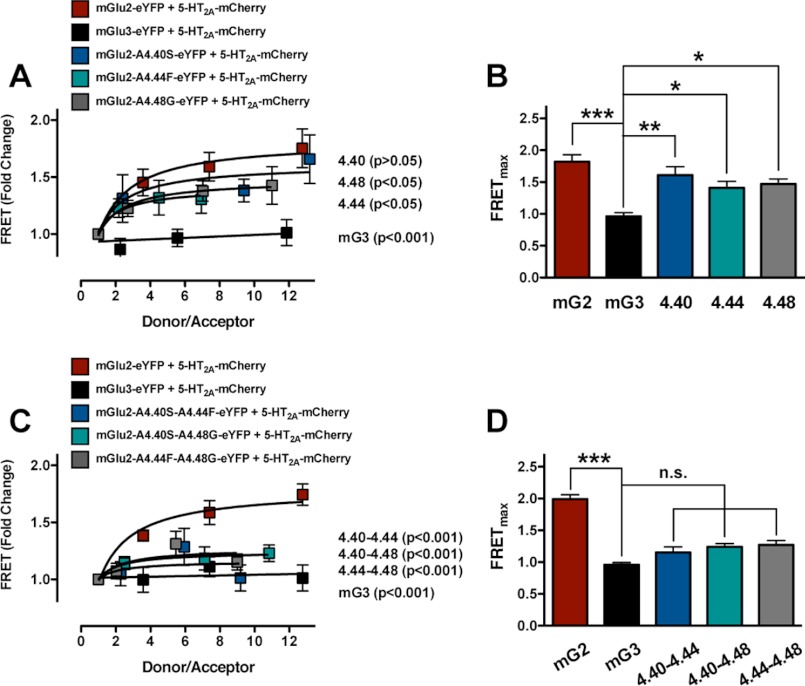
FIGURE 6.
Any two of the three residues located at the intracellular end of TM4 of mGlu2 mediate complex formation with the 5-HT2A receptor. A, fold change of FCM-based FRET signal for each combination of eYFP- or mCherry-tagged receptors (eYFP-tagged, donor; mCherry-tagged, acceptor). Data obtained in cells co-expressing either mGlu2, mGlu3, mGlu2-A677S4.40, mGlu2-A681F4.44, or mGlu2-A685G4.48, all tagged with eYFP, and 5-HT2A-cerulean can be fit preferably by a saturation curve, assessed by F test. Data obtained in cells co-expressing mGlu3-eYFP and 5-HT2A-mCherry show linear correlations (n = 4–8). Note that mGlu2-A681F4.44, mGlu2-A685G4.48, or mGlu3, but not mGlu2-A677S4.40, decrease the FCM-based FRET signal as compared with mGlu2: mGlu2-eYFP + 5-HT2A-mCherry compared with mGlu3-eYFP + 5-HT2A-mCherry, F(2,40) = 40.17, p < 0.001; mGlu2-eYFP + 5-HT2A-mCherry compared with mGlu2-A677S4.40-eYFP + 5-HT2A-mCherry, F(2,28) = 0.76, p > 0.05; mGlu2-eYFP + 5-HT2A-mCherry compared with mGlu2-A681F4.44-eYFP + 5-HT2A-mCherry, F(2,44) = 3.62, p < 0.05; mGlu2-eYFP + 5-HT2A-mCherry compared with mGlu2-A685G4.48-eYFP + 5-HT2A-mCherry: F(2,36) = 3.70, p < 0.05. B, FRETmax obtained from individual FCM-based FRET saturation curves. Note that FRETmax is significantly increased in mGlu2, mGlu2-A677S4.40, mGlu2-A681F4.44, and mGlu2-A685G4.48 as compared with mGlu3. **, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant; Bonferroni's post hoc test of one-way ANOVA. C, fold change of FCM-based FRET signal for each combination of eYFP- or mCherry-tagged receptors (eYFP-tagged, donor; mCherry-tagged, acceptor). Data obtained in cells co-expressing either mGlu2, mGlu2-A677S4.40/A681F4.44, mGlu2-A677S4.40/A685G4.48, or mGlu2-A681F4.44/A685G4.48, all tagged with eYFP, and 5-HT2A-mCherry can be fit preferably by a saturation curve, assessed by F test. Data obtained in cells co-expressing mGlu3-eYFP and 5-HT2A-mCherry show linear correlations (n = 8–23). Note that mGlu2-A677S4.40/A681F4.44, mGlu2-A677S4.40/A685G4.48, mGlu2-A681F4.44/A685G4.48, or mGlu3 all decrease the FCM-based FRET signal as compared with mGlu2: mGlu2-eYFP + 5-HT2A-mCherry compared with mGlu3-eYFP + 5-HT2A-mCherry, F(2,150) = 52.28, p < 0.001; mGlu2-eYFP + 5-HT2A-mCherry compared with mGlu2-A677S4.40/A681F4.44-eYFP + 5-HT2A-mCherry, F(2,106) = 14.96, p < 0.001; mGlu2-eYFP + 5-HT2A-mCherry compared with mGlu2-A677S4.40/A685G4.48-eYFP + 5-HT2A-mCherry, F(2,118) = 18.02, p < 0.001; mGlu2-eYFP + 5-HT2A-mCherry compared with mGlu2-A681F4.44/A685G4.48-eYFP + 5-HT2A-mCherry, F(2,121) = 13.97, p < 0.001. D, FRETmax obtained from individual FCM-based FRET saturation curves. Note that FRETmax is significantly increased in mGlu2, but not in mGlu2-A677S4.40/A681F4.44, mGlu2-A677S4.40/A685G4.48, and mGlu2-A681F4.44/Ala-685G4.48, as compared with mGlu3. ***, p < 0.001; n.s., not significant; Bonferroni's post hoc test of one-way ANOVA. Error bars represent S.E.