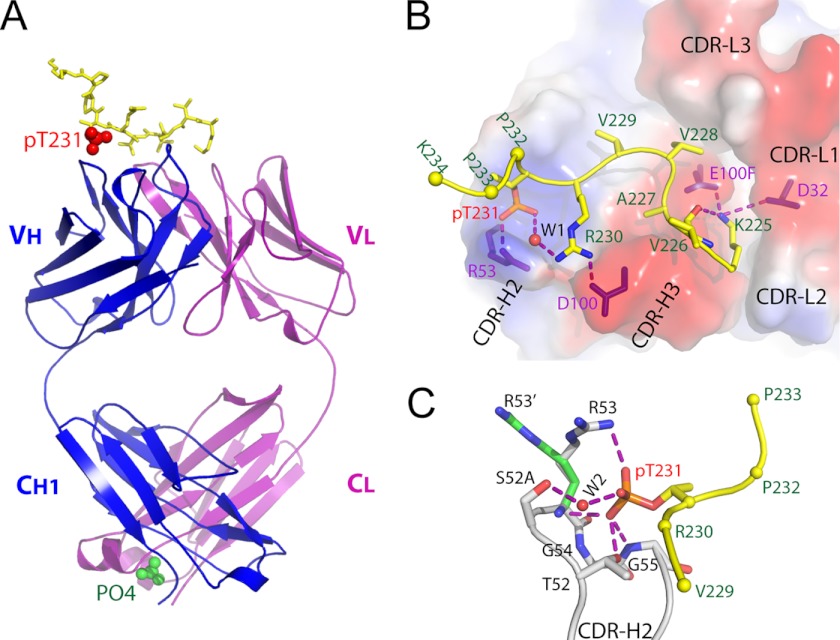
FIGURE 3.
Structure of anti-phospho-tau Fab (pT231/pS235_1) in complex with phosphoepitope pT231/pS235. A, schematic view showing the complex. The Fab heavy chain (VH + CH1) and light chain (VL + CL) are shown in blue and purple, respectively. The phosphoepitope is shown as yellow sticks on top with phosphorylated Thr-231 (pT231) highlighted as red spheres. The solvent PO43− ion is labeled and shown as green spheres. B, transparent electrostatic surface view of the CDRs showing the strong electrostatic interactions between CDR residues and the phosphoepitope. Positively charged areas are shown in blue, and negatively charged areas are shown in red. The backbone of the bound phosphoepitope is shown as a schematic in yellow; the side chains are shown as a stick model in atomic colors (carbon, yellow; nitrogen, blue; oxygen, red; and phosphorus, orange). The side chains of Pro-232, Pro-233, and Lys-234 are omitted for clarity, with Cα shown as spheres. The residues within CDRs that form strong electrostatic interactions with the phosphoepitope are shown as purple sticks. A water molecule is labeled W1. Hydrogen bonds are shown as dashed purple lines. C, interaction details between CDR-H2 and pThr-231. Two conformations of the H/Arg-53 side chain are shown as R53 and R53′, respectively. Hydrogen bonds are shown as dashed purple lines. A water molecule is labeled W2.