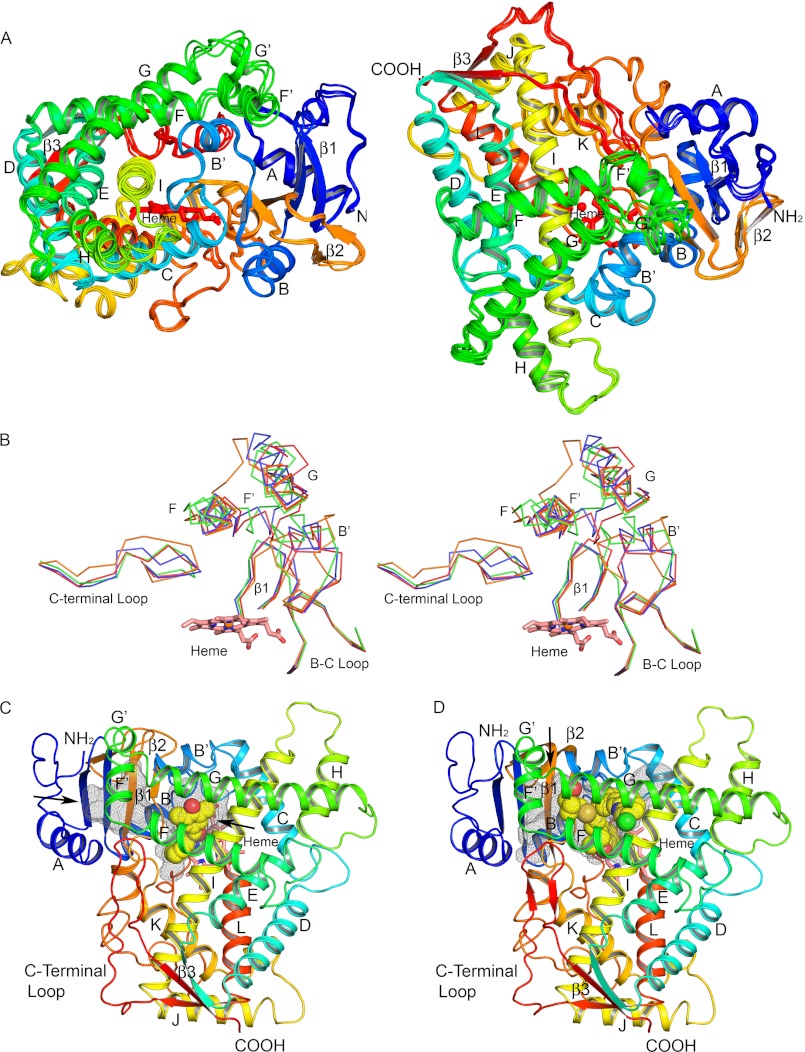
FIGURE 2.
Secondary and tertiary structure of P450 2C19. A, four chains in the asymmetric unit are superimposed, and each chain is colored from blue at the N terminus to red at the C terminus. Helices are designated by letters, and sheets are numbered. The heme prosthetic group is depicted as a stick figure colored red. The chains differ most extensively between helices F′ and G′ and adjacent regions. These conformational differences can be attributed to distinct lattice packing interactions for each chain that can restrain flexible regions to different extents in the crystal. B, traces for Cα atoms of divergent regions evident when the structures of P450s 2C8, PDB code 2NNI chain A (blue trace), 2C9, PDB code 1R9O (orange trace), and 2C9m7, PDB code 1OG2 chain A (green trace), are overlaid on the structure of chain A of the P450 2C19 0XV complex (red trace). Side-by-side images are shown for cross-eyed stereo viewing. C, structure of the P450 2C19 0XV complex has two internal cavities depicted as mesh surfaces. 0XV is rendered as atomic spheres with carbons and oxygens colored yellow, and red occupies the active site cavity above the surface of the heme. A second cavity, which may serve as the substrate access channel, is located under helix F′ and above sheet β-1. D, in contrast, the active site of P450 2C8 (PDB code 2NNI) encompasses both cavities seen in the structure of the P450 2C19 0XV complex. Montelukast, shown as atomic spheres with carbons colored yellow, occupies three branches of the larger active site cavity. Oxygen, nitrogen, chlorine, iron, and sulfur atoms are colored red, blue, green, orange, and gold, respectively. Arrows indicate open solvent channels to the interior of the protein in C and D.