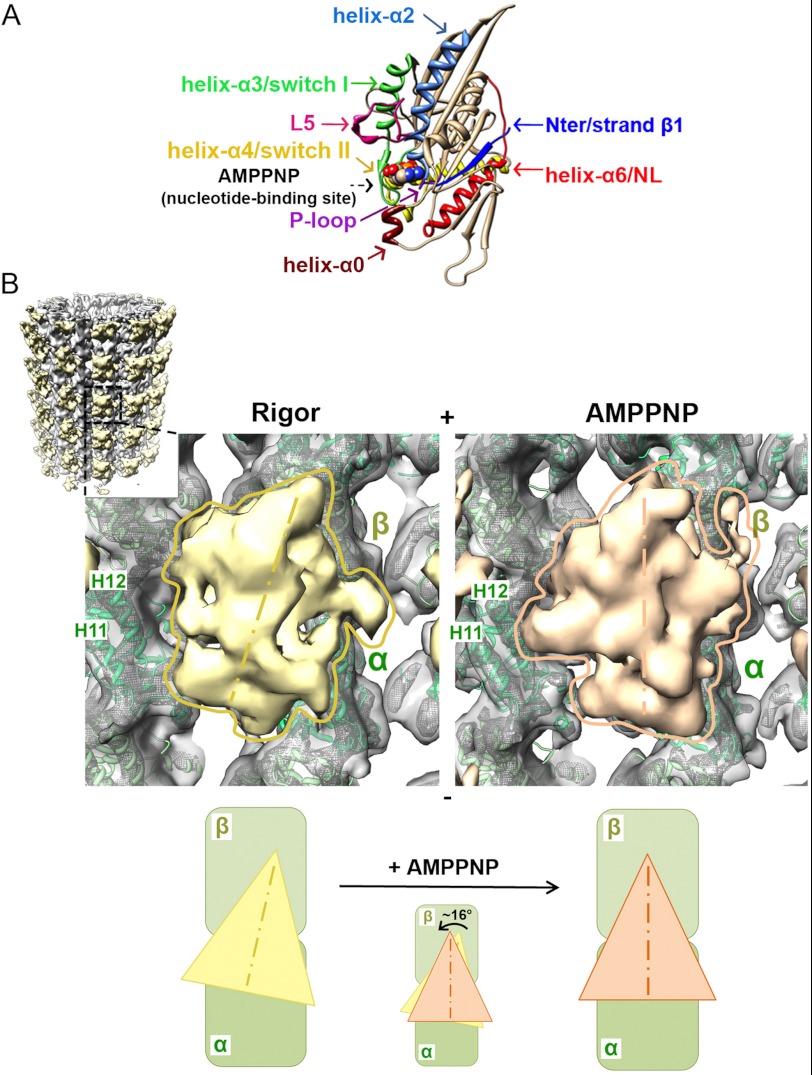
FIGURE 1.
Cryo-EM reconstructions of human K5 MD before and after ATP binding. A, crystal structure of arrowhead-shaped K5 MD bound to AMPPNP viewed with the MT-binding surface at the back. The mechanochemical elements in the MD that sense and respond to bound nucleotide are color-coded and labeled with arrows, with AMPPNP shown in space-fill representation (PDB entry 3HQD (20)). B, cryo-EM reconstructions of MT-bound human K5 MD in rigor (left) and AMPPNP (right) states. The inset shows the whole rigor reconstruction of MT-bound K5 MD, whereas the main panels show a single K5 MD bound to αβ-tubulin dimer (surface contoured at 1.1 σ and mesh contoured at 4 σ, with green ribbon docked) in rigor (yellow) and AMPPNP (orange). Helices H11 and H12 in α-tubulin are labeled. The schematic below illustrates the rotation of the K5 MD that occurs when AMPPNP binds. The MT plus-end is toward the top of this and all subsequent figures.