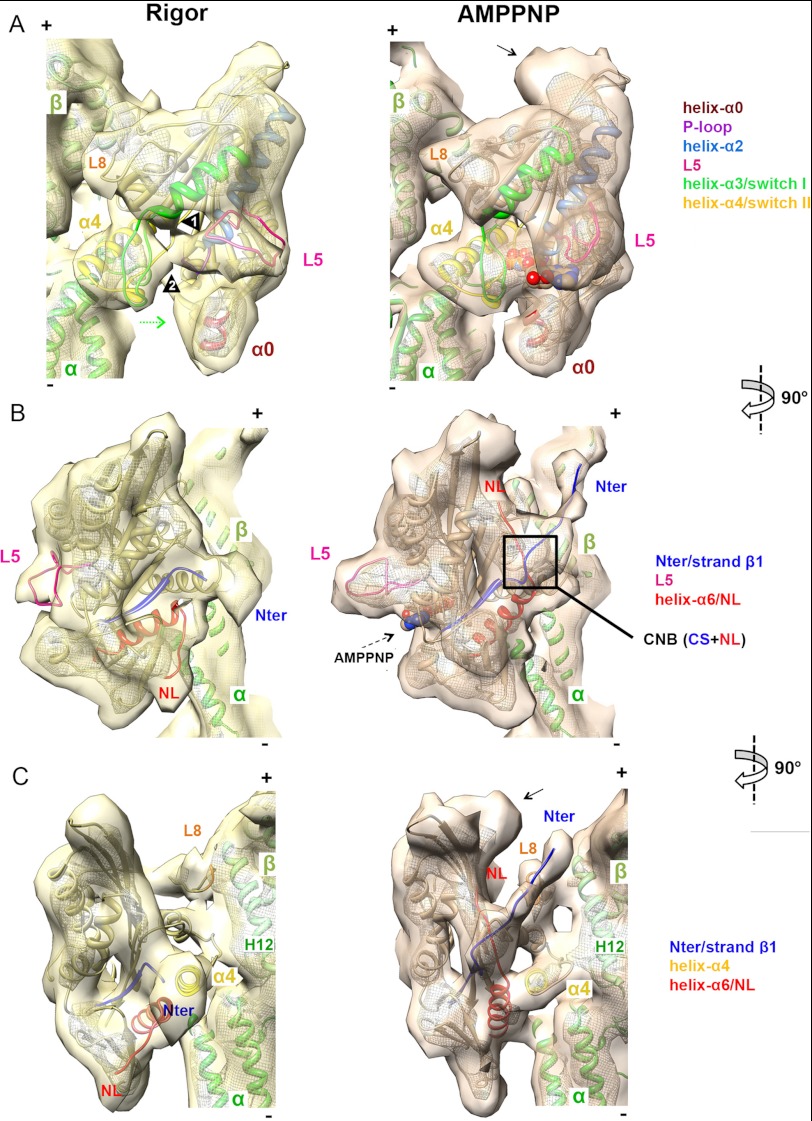
FIGURE 2.
Pseudoatomic models of nucleotide-dependent conformational changes of MT-bound K5. A–C, orthogonal views of a single αβ-tubulin dimer with K5 MD in rigor (left, in yellow) and AMPPNP (right, in orange) reconstructions, contoured as in Fig. 1B. The docked pseudoatomic coordinates are color-coded according to the key. A, view of the nucleotide-binding pocket. Arrowheads 1 and 2 indicate L5 interaction with helix-α3 and switch I, respectively. The curved density at the plus-end MD tip, not accounted for by docked coordinates in the AMPPNP reconstruction (indicated by an arrow), probably corresponds to additional NL residues and part of the histidine tag of our construct; it is only visible when the NL is docked and was previously observed in a K3-AMPPNP MT-bound reconstruction with an equivalent affinity tag (49). B, view of the front face of the K5 MD. C, the view of the NL clearly shows NL docking and ordering of the N terminus.