FIGURE 1.
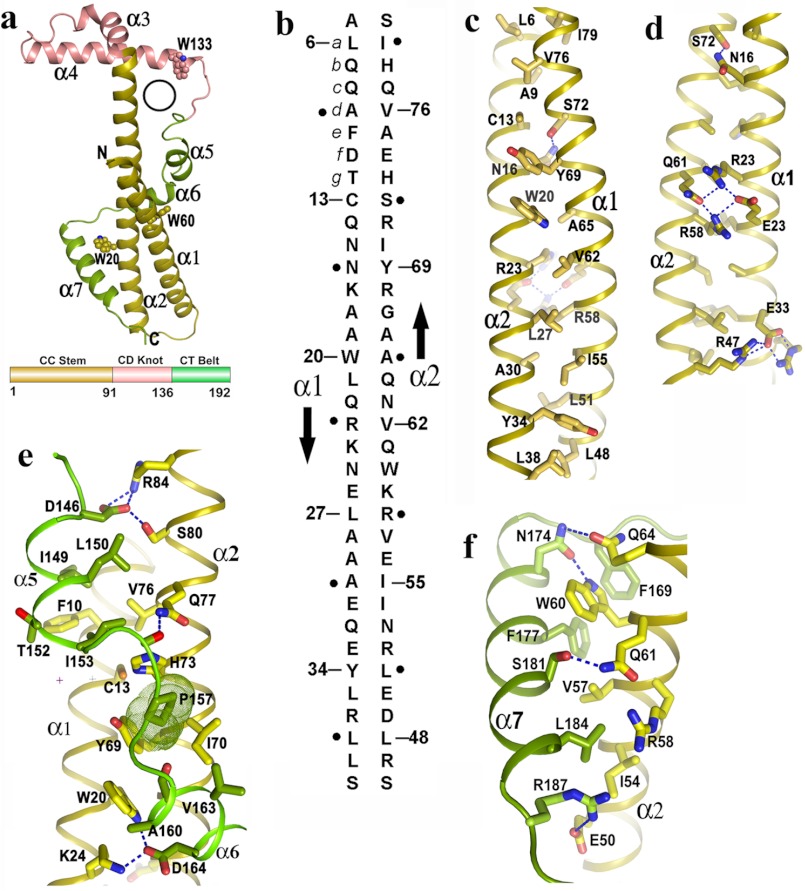
Novel fold of Psu and stabilization of its coiled-coil region. a, schematic representation of Psu monomer resembling the structure of a golf stick, seven helices and three Trp residues are labeled. A black circle represents the hole in one monomer through which α3 of the second monomer can pass (top), topological diagram of Psu showing different regions (bottom) (same color scheme is used in c–f). b, schematic representation of antiparallel two stranded coiled-coil in Psu. The amino acid sequence of α1 and α2 are shown vertically, and the arrows indicate the chain direction. Residues at position a of each heptad repeat are numbered, whereas residues at position d are indicated as black dot. c, hydrophobic core of the CC stem formed by residues a of one helix with residues d of the other. d, view 180° rotated of c along the coiled-coil axis showing the polar/charged interactions that stabilize the CC stem. e, clamping of α5 and α6 with coiled coil. Pro-157, shown as dotted surface, packs with Cys-13, Tyr-69, Ile-57, His-73. f, interactions of α7 with α2.