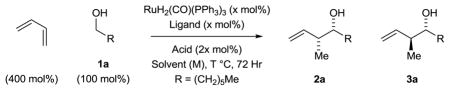
Table 1.
Selected optimization experiments in the ruthenium catalyzed syn-diastereo- and enantioselective hydrohydroxyalkylation of butadiene with heptanol 1a.a
Yields are of material isolated by silica gel chromatography. DPPF = 1,1′-bis(diphenylphosphino)ferrocene; SEGPHOS = 5,5′-
bis(diphenylphosphino)-4,4′-bi-1,3-benzodioxole. See Supporting Information for further details.