Abstract
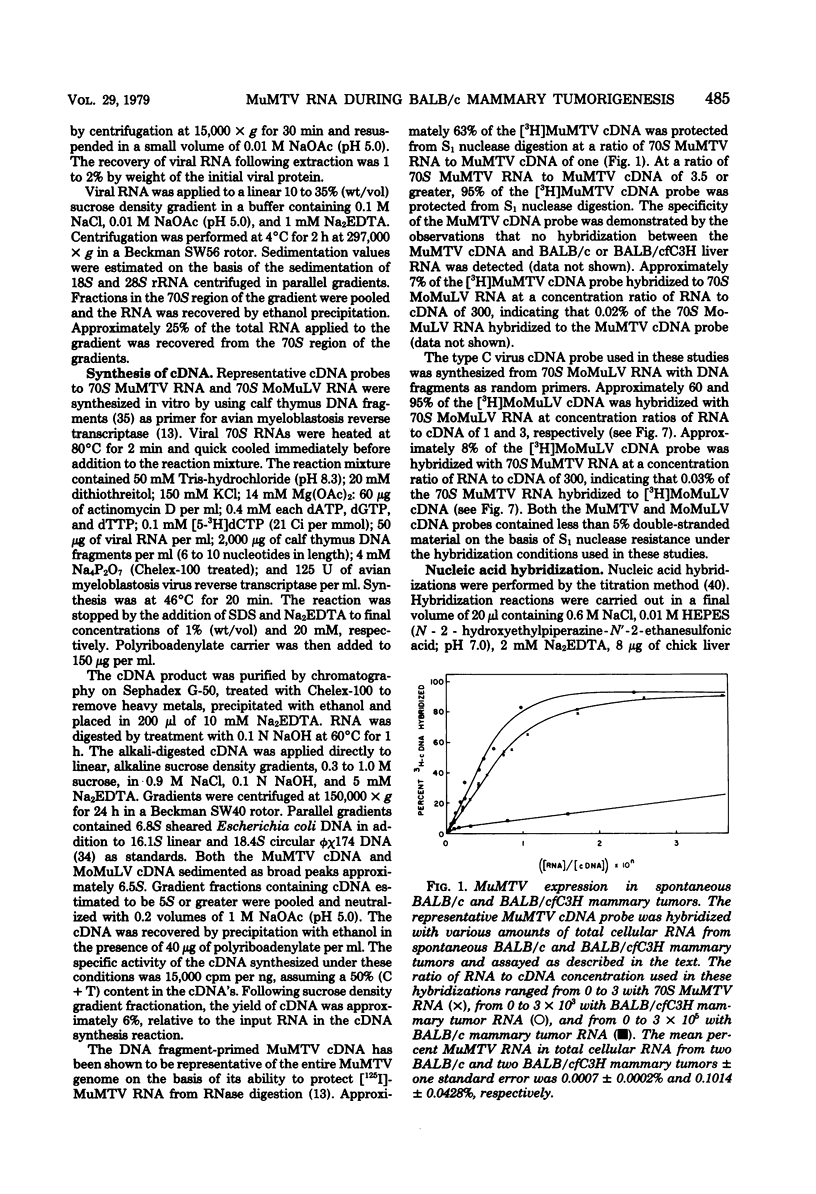
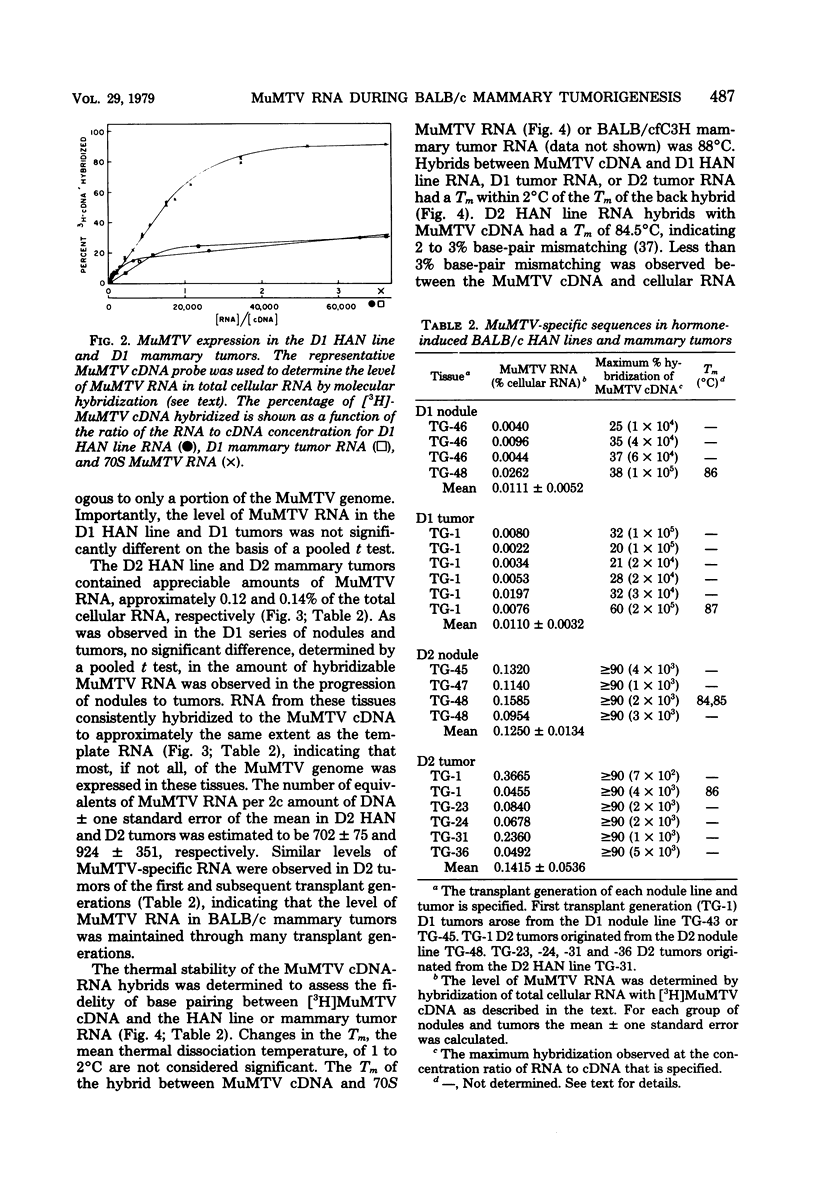
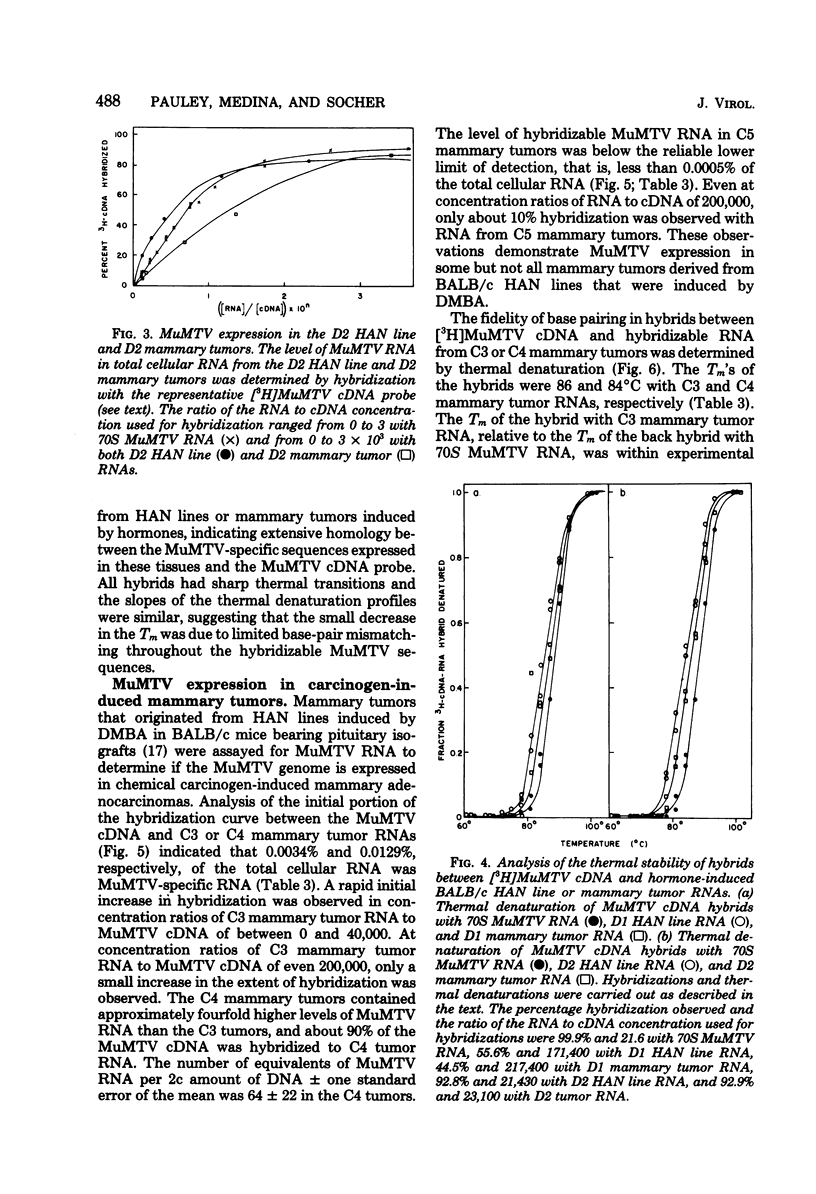
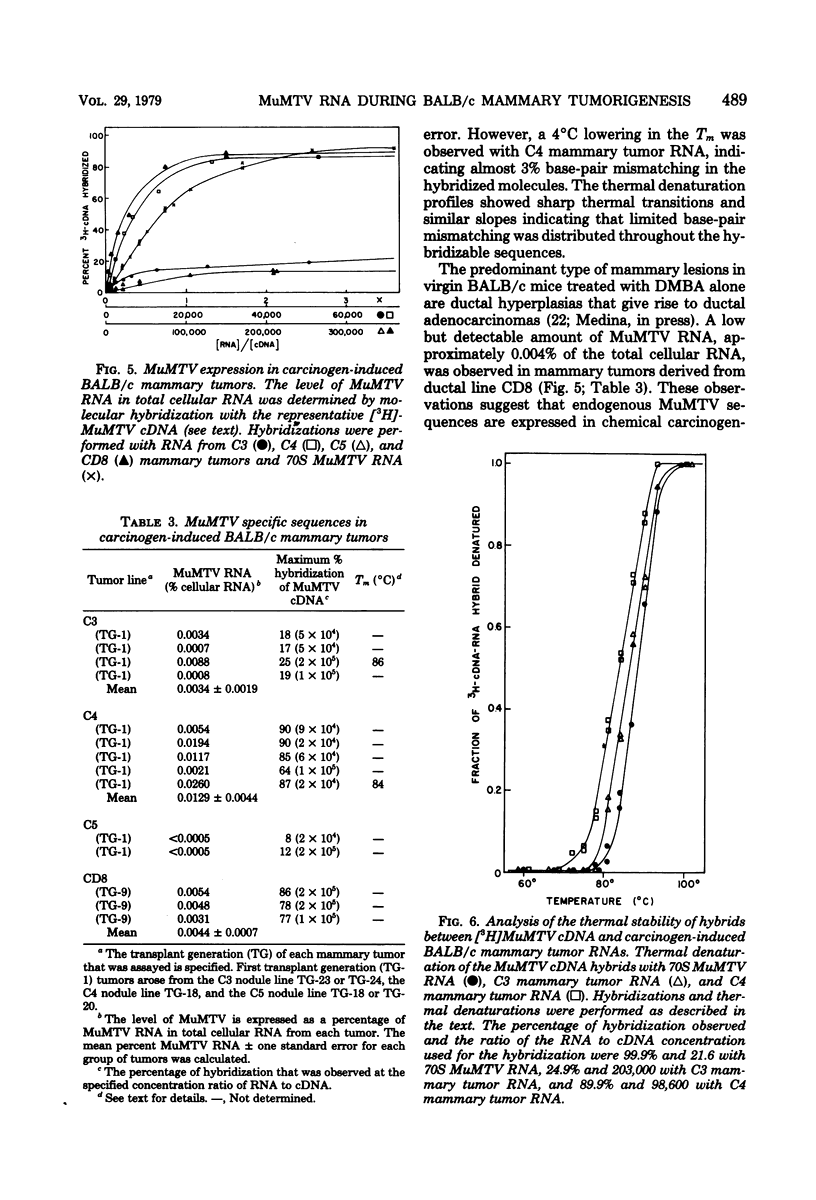
Steady-state levels of murine mammary tumor virus (MuMTV) RNA were quantitated during mammary tumorigenesis in BALB/c mice by molecular hybridization with a representative MuMTV complementary DNA (cDNA) probe. Hyperplastic alveolar nodule (HAN) lines are preneoplastic mammary lesions that were induced in BALB/c mice by hormones alone or in combination with 7,12-dimethylbenz(a)anthracene and give rise to mammary tumors. The hormone-induced HAN lines D1 and D2 contained detectable amounts of hybridizable MuMTV sequences. MuMTV RNA sequences were also observed in five of the six transplanted BALB/c mammary tumors that were examined. Similar levels of hybridizable MuMTV RNA were observed between the D1 or D2 HAN line and mammary tumors derived from each HAN line. The D2 HAN line as well as D2, C4, and CD8 mammary tumors accumulated RNA that was apparently homologous to most of the MuMTV genome. Thermal denaturation of hybrids indicated extensive sequence homology between the MuMTV cDNA and hybridizable RNA in the BALB/c HAN lines and mammary tumors. A low level of type C viral RNA was observed in the BALB/c HAN lines and most mammary tumors by molecular hybridization with a cDNA to Moloney murine leukemia virus. These data demonstrate that MuMTV sequences are frequently expressed in hormone-induced BALB/c HAN lines and mammary tumors derived from HAN lines or ductal hyperplasias induced in BALB/c mice by hormones and/or a chemical carcinogen. The transition from the preneoplastic to the neoplastic state in BALB/c mice does not appear to be due to a change in the steady-state levels of MuMTV RNA since the hormone-induced HAN lines and mammary tumors had similar levels of hybridizable MuMTV RNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentvelzen P. Endogenous mammary tumor viruses in mice. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1145–1150. doi: 10.1101/sqb.1974.039.01.131. [DOI] [PubMed] [Google Scholar]
- Bentvelzen P. Host-virus interactions in murine mammary carcinogenesis. Biochim Biophys Acta. 1974 Dec 31;355(3-4):236–259. doi: 10.1016/0304-419x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEOME K. B., BLAIR P. B., FAULKIN L. J., Jr Some characteristics of the preneoplastic hyperplastic alveolar nodules of C3H/Crgl mice. Acta Unio Int Contra Cancrum. 1961;17:973–982. [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- Dion A. S., Heine U. I., Pomenti A. A., Korb J., Weber G. H. Electrophoretic analysis of the molecular weight of murine mammary tumor virus RNA. J Virol. 1977 Jun;22(3):822–825. doi: 10.1128/jvi.22.3.822-825.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Vaidya A. B., Fout G. S. Cation preferences for poly(rC)-oligo(dG)-directed DNA synthesis by RNA tumor viruses and human milk particulates. Cancer Res. 1974 Dec;34(12):3509–3515. [PubMed] [Google Scholar]
- Drohan W., Kettmann R., Colcher D., Schlom J. Isolation of the mouse mammary tumor virus sequences not transmitted as germinal provirus in the C3H and RIII mouse strains. J Virol. 1977 Mar;21(3):986–995. doi: 10.1128/jvi.21.3.986-995.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. P., Butel J. S., Socher S. H., Rosen J. M. Detection of mouse mammary tumor virus RNA in BALB/c tumor cell lines of nonviral etiologies. J Virol. 1978 Dec;28(3):743–752. doi: 10.1128/jvi.28.3.743-752.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete E. A comparative morphological study of the mammary gland in a high and a low tumor strain of mice. Am J Pathol. 1938 Sep;14(5):557–578.5. [PMC free article] [PubMed] [Google Scholar]
- GARDNER W. U. Hormonal aspects of experimental tumorigenesis. Adv Cancer Res. 1953;1:173–232. doi: 10.1016/s0065-230x(08)60004-4. [DOI] [PubMed] [Google Scholar]
- McGrath C. M., Marineau E. J., Voyles B. A. Changes in MuMTV DNA and RNA levels in Balb/c mammary epithelial cells during malignant transformation by exogenous MuTV and by hormones. Virology. 1978 Jun 15;87(2):339–353. doi: 10.1016/0042-6822(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Medina D., DeOme K. B. Carcinogen-induced mammary tumors from preneoplastic nodule outgrowths in BALB-c mice. Cancer Res. 1970 Apr;30(4):1055–1059. [PubMed] [Google Scholar]
- Medina D., DeOme K. B. Effects of various oncogenic agents on tumor-producing capabilities of series D BALB-c mammary nodule outgrowth lines. J Natl Cancer Inst. 1970 Aug;45(2):353–363. [PubMed] [Google Scholar]
- Medina D., DeOme K. B. Influence of mammary tumor virus on the tumor-producing capabilities of nodule outgrowth free of mammary tumor virus. J Natl Cancer Inst. 1968 Jun;40(6):1303–1308. [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. VI. Tumor-producing capabilities of mammary dysplasias in BALB/cCrgl mice. J Natl Cancer Inst. 1976 Nov;57(5):1185–1189. doi: 10.1093/jnci/57.5.1185. [DOI] [PubMed] [Google Scholar]
- Medina D. Preneoplastic lesions in murine mammary cancer. Cancer Res. 1976 Jul;36(7 Pt 2):2589–2595. [PubMed] [Google Scholar]
- Medina D., Warner M. R. Mammary tumorigenesis in chemical carcinogen-treated mice. IV. Induction of mammary ductal hyperplasias. J Natl Cancer Inst. 1976 Aug;57(2):331–337. doi: 10.1093/jnci/57.2.331. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Pauley R. J., Rosen J. M., Socher S. H. Mammary tumour virus and casein gene transcription during mouse mammary development. Nature. 1978 Oct 5;275(5679):455–457. doi: 10.1038/275455a0. [DOI] [PubMed] [Google Scholar]
- Rosen J. M. Isolation and characterization of purified rat casein messenger ribonucleic acids. Biochemistry. 1976 Nov 30;15(24):5263–5271. doi: 10.1021/bi00669a011. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schlom J., Colcher D., Drohan W., Kettmann R., Michalides R., Vlahakis G., Young J. Differences in mouse mammary tumor viruses. Relationship to early and late occurring mammary tumors. Cancer. 1977 Jun;39(6 Suppl):2727–2733. doi: 10.1002/1097-0142(197706)39:6<2727::aid-cncr2820390660>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Schlom J., Michalides R., Kufe D., Hehlmann R., Spiegelman S., Bentvelzen P., Hageman P. A comparative study of the biologic and molecular basis of murine mammary carcinoma: a model for human breast cancer. J Natl Cancer Inst. 1973 Aug;51(2):541–551. [PubMed] [Google Scholar]
- Smith G. H., Pauley R. J., Socher S. H., Medina D. Chemical carcinogenesis in C3H/StWi mice, a worthwhile experimental model for breast cancer. Cancer Res. 1978 Dec;38(12):4504–4509. [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Teramoto Y. A., Puentes M. J., Young L. J., Cardiff R. D. Structure of the mouse mammary tumor virus: polypeptides and glycoproteins. J Virol. 1974 Feb;13(2):411–418. doi: 10.1128/jvi.13.2.411-418.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. The relationship between mismatched base pairs and the thermal stability of DNA duplexes. I. Effects of depurination and chain scission. Biochim Biophys Acta. 1973 Feb 4;294(1):405–415. doi: 10.1016/0005-2787(73)90095-6. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Young B. D., Harrison P. R., Gilmour R. S., Birnie G. D., Hell A., Humphries S., Paul J. Kinetic studies of gene frequency. II. Complexity of globin complementary DNA and its hybridization characteristics. J Mol Biol. 1974 Apr 25;84(4):555–568. doi: 10.1016/0022-2836(74)90116-8. [DOI] [PubMed] [Google Scholar]


