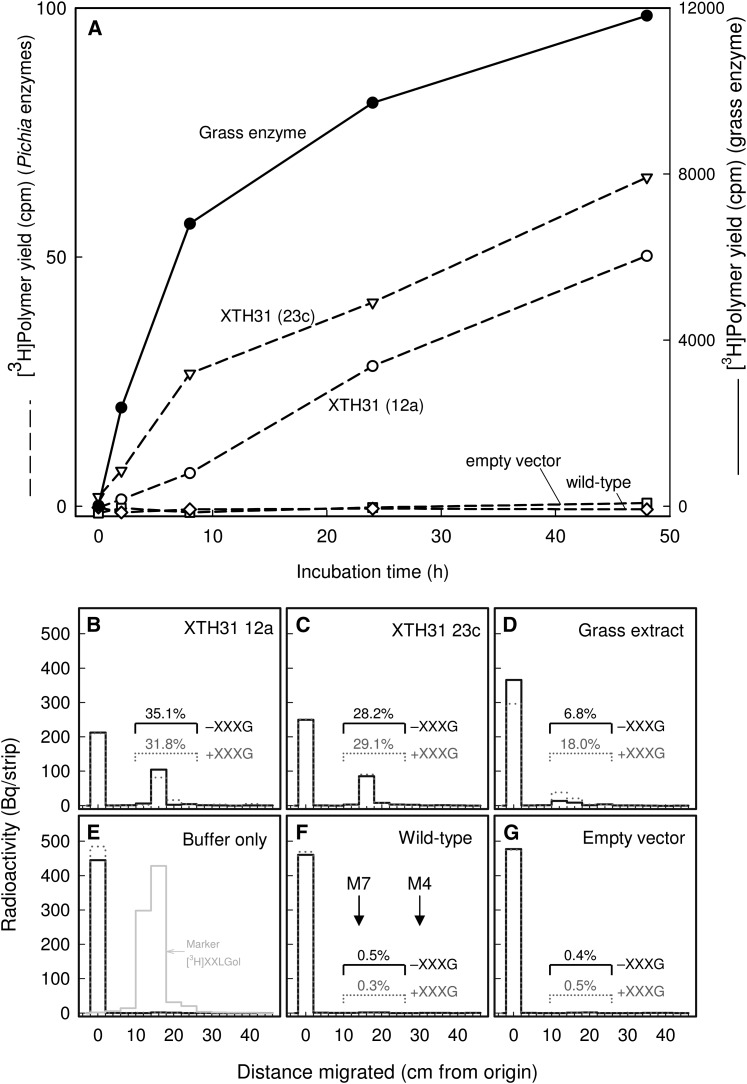
Figure 5.
P. pastoris Cultures Expressing XTH31 Produce Slight XET but High XEH Activity.
(A) Standard radiochemical XET assay. Proteins secreted by XTH31-transformed P. pastoris cells (lines 12a and 23c), wild-type cells, or cells transformed with empty vector were tested by a sensitive radiochemical assay for XET activity (dashed lines). A crude extract of the grass H. lanatus was tested under identical conditions (solid line; right-hand scale).
(B) to (G) Novel assay for XEH and XET activity. The same five protein preparations ([B], XTH31 from P. pastoris line 12a; [C], XTH31 from P. pastoris line 23c; [D], H. lanatus; [F], wild-type P. pastoris; [G], P. pastoris transformed with empty vector), and a buffer-only control (E) were incubated with [reducing-end-3H]xyloglucan in the absence (solid line) or presence (dotted line) of 200 µM nonradioactive XXXG. After 48 h of incubation, the products were analyzed by paper chromatography, revealing any free 3H-oligosaccharides generated by the enzyme (∼15 cm from origin) and the remaining [3H]xyloglucan (0 cm from origin). The yields of 3H-oligosaccharides as a percentage of total 3H are indicated above each enzyme’s profile. (E) also shows the profile of authentic free [3H]XXLGol (gray histogram), which is expected to be the major oligosaccharide released. (F) shows the positions of the external markers maltoheptaose (M7) and maltotetraose (M4).