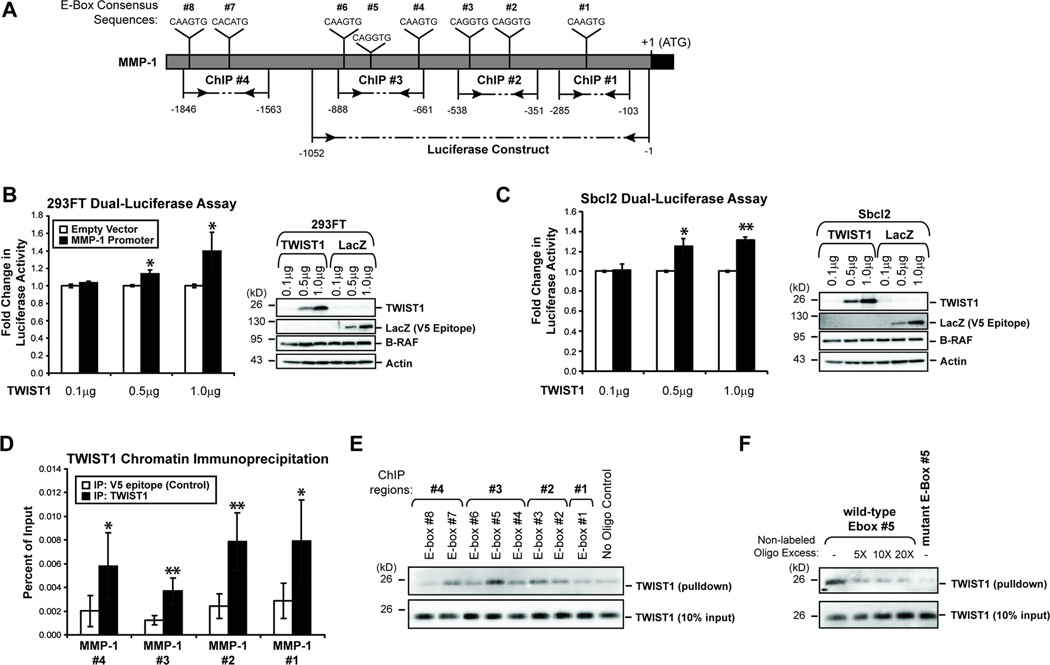
Figure 5.
TWIST1 alters MMP-1 promoter activity in a dose-dependent manner and directly binds the MMP-1 promoter. (A) A schematic of the MMP-1 promoter highlighting the regions utilized in the dual-luciferase assay and ChIP assay, as well as the E-box consensus sequences (CANNTG) present. (B) A dual-luciferase assay was performed to determine whether the MMP-1 promoter activity would change as a result of increasing TWIST1 expression in 293FT cells. Fold change was calculated by dividing the normalized luciferase activities of TWIST1-expressing 293FT cells by the normalized luciferase activity of LacZ-expressing cells. A western blot confirmed differential expression of TWIST1 and LacZ. Columns, average of three independent experiments; bars, SD; *p<0.05. (C) A dual-luciferase assay was performed in Sbcl2 melanoma cells as above. Columns, average of three independent experiments; bars, SD; *p<0.05, **p<0.01. (D) In parental WM793 cells, ChIP followed by quantitative PCR was performed to determine if TWIST1 binds directly to the MMP-1 promoter. V5 epitope immunoprecipitation was performed as a negative control for enrichment. Columns, average of six independent experiments; bars, SD; *p<0.05, **p<0.01. (E) A biotinylated oligonucleotide pulldown assay was performed with whole cell protein lysates from parental WM793 cells. A pulldown without oligonucleotide was performed as a negative control. Following the pulldown, western blots were conducted to determine TWIST1 enrichment relative to 10% input. (F) Oligonucleotide pulldowns were repeated for MMP-1 E-box #5 without non-labeled competitor, or in the presence of 5-, 10-, and 20-fold excess of non-labeled E-box #5 competitor. Also, a biotinylated mutant E-box #5 oligonucleotide (4 mutant nucleotides) was utilized to determine the specificity of the interaction.