Abstract
In myasthenia gravis (MG) and experimental autoimmune MG (EAMG), many pathologically significant autoantibodies are directed to the main immunogenic region (MIR) of muscle nicotinic acetylcholine receptors (AChRs), a conformation-dependent region at the extracellular tip of α1 subunits of AChRs. Human muscle AChR α1 MIR sequences were integrated into Aplesia ACh-binding protein (AChBP). The chimera potently induced EAMG. AChBP induced EAMG much less potently. AChBP is a water-soluble protein resembling the extracellular domain of AChRs, yet rats immunized with chimeras developed autoantibodies to both extracellular and cytoplasmic domains of muscle AChRs. We propose that an initial autoimmune response directed at the MIR leads to an autoimmune response sustained by muscle AChRs. Autoimmune stimulation sustained by endogenous muscle AChR may be a target for specific immunosuppression. These studies show that the α1 MIR is highly myasthenogenic, and that AChR-like proteins distantly related to muscle AChR can induce EAMG and, potentially, MG.
Keywords: nicotinic acetylcholine receptor, AChR, MG, EAMG, antigenic structure
The main immunogenic region (MIR) is a conformation-dependent region at the extracellular tip of muscle AChR α1 subunits.1–3 It is defined by the competitive binding of monoclonal antibodies (mAbs). It is the target of half or more of autoantibodies in myasthenia gravis (MG) and its animal model experimental autoimmune myasthenia gravis (EAMG). EAMG is typically induced by immunization with muscle-like AChRs from the electric organs of Torpedo californica.2 Monoclonal antibodies (mAbs) to the MIR made from rats and mice with EAMG have all of the pathological activities of serum antibodies to AChRs: they can bind to the extracellular surface of muscle AChRs in vivo, fix complement causing focal lysis and the acute form of EAMG, crosslink muscle AChRs triggering their lysosomal destruction (antigenic modulation), and passively transfer EAMG.1–3
MIR chimeras
Detailed antigenic structure of the MIR was determined using chimeras of human α1 subunit sequence in Aplysia californica acetylcholine binding protein (AChBP) and human α7 AChR.3 AllAChR subunits have homologous structures. The muscle-like AChRs of the electric organ of Torpedo californica have five subunits organized like barrel staves in the order α1, γ, α1, δ, β1 to form a central cation channel across the membrane whose opening is controlled by two ACh binding sites at the interfaces of α1 with γ and δ subunits.4 AChBP has five identical subunits with five ACh binding sites at their interfaces.5 AChBP resembles the extracellular structure of an AChR. AChBP subunits lack the transmembrane and cytoplasmic domains of AChR subunits, consequently AChBPs are soluble proteins. They are secreted by mollusk glia to modulate cholinergic signaling. There is no vertebrate homologue. Because AChBPs are water soluble, they are easy to crystallize, so their structure is known in great detail from X-ray crystallography.5 AChBPs provide a model for the extracellular domains of AChRs and related receptors that are very difficult to crystallize.
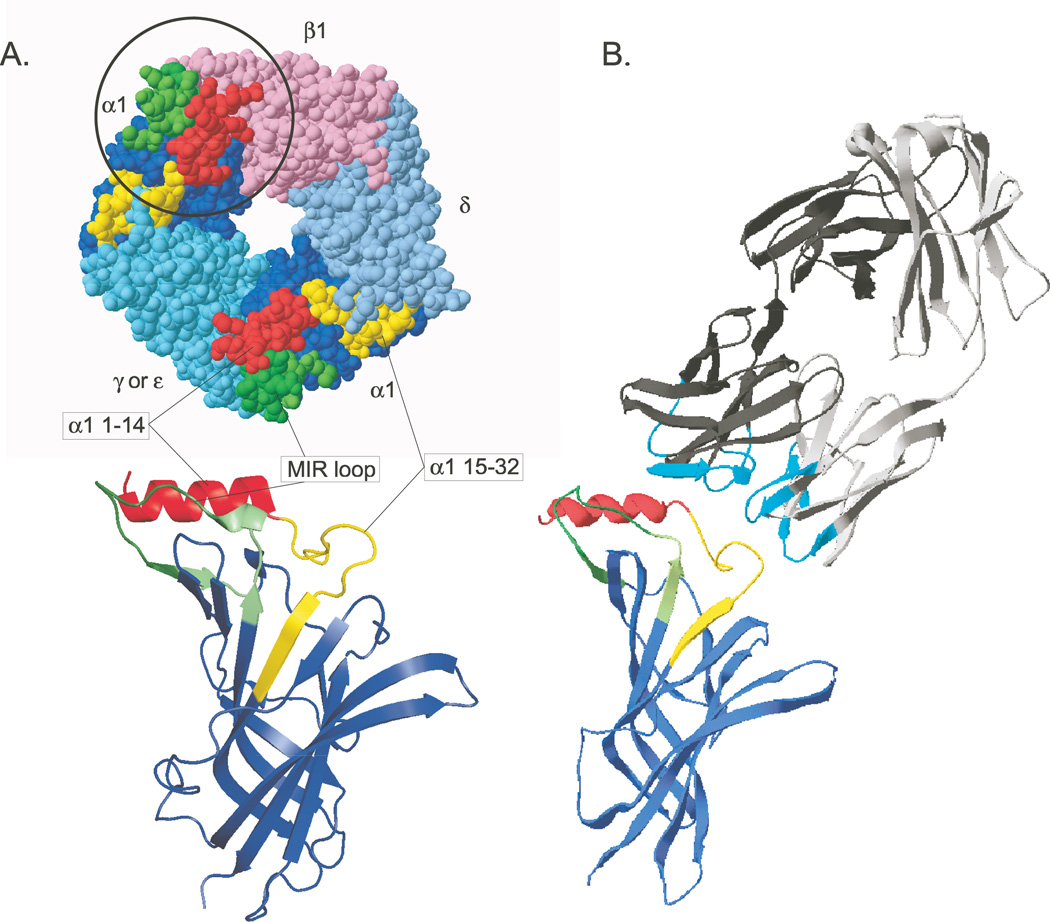
Chimeras in which human α1 subunit sequences replace homologous parts of the AChBP protein insure that the α1 sequences assume conformations similar or identical to their conformation in native α1 subunits.3 In order to make chimeras with AChBP or α7 AChRs that exhibited high affinity for four mAbs to the MIR derived from rats with EAMG and one mAb derived from a human with MG, it was necessary to include two α1 sequences: the N-terminal α helix (1–14) and the MIR loop (67–76).3 The interaction between these two sequences accounts for the dependence of the antigenicity of the MIR on its native conformation. Some additional sequence was also required to provide additional components of the adjacent and overlapping epitopes that form the MIR or to permit its proper conformation in the chimera. The chimera α1 (1–30, 60–81)/AChBP exhibited KD values for the mAbs of 0.075–2.5 nM and bound to serum autoantibodies from human, canine, and feline MG patients.3 The human MG mAb bound to native but unassembled human α1 subunits with a KD = 70 nM, to assembled human muscle AChRs with a KD = 0.0068 nM, and to the chimera with a KD = 0.075 nM. Figure 1 depicts the structure of the chimera, a chimeric subunit, a model of the extracellular domain of the α1 subunit, and the structure of a Fab fragment of an MIR mAb.
Figure 1.
A model of the MIR α1(1–32, 60–81)/AChBP chimera and its interaction with an antibody to the MIR is shown. (A) MIR components are highlighted on a top view of the crystal structure of an Aplysia AChBP subunit.15 Below, a front view ribbon diagram shows a single chimeric subunit. (B) The crystal structure of Fab 19216 is accompanied by the structure of the mouse α1 extracellular domain.17 Small differences in the sequences and conformation of the epitopes within the MIR profoundly influence the affinity with which antibodies are bound. The large size of bivalent IgG molecules with respect to the size of the MIR can result in competitive binding between different closely spaced epitopes within the MIR. The six hypervariable loops of the Fab, which form its antigen binding site are highlighted in cyan. This unusual mAb to the MIR does not appear to bind to the MIR loop per se, but competes for binding with mAbs, which do. The Fab is angled to suggest this, but not actually docked on the subunit model. This is part of Figure 1 from Luoet et al.3
Interaction between the N-terminal α helix and the MIR loop may be a critical step in the conformational maturation of AChR subunits that allows their assembly into mature AChRs. For example, α7 AChR subunits are efficiently synthesized in many cells, but their conformational maturation and consequent ability to assemble into mature AChRs depends on the chaperone Ric-3 (Ref. 6). By contrast, α1 subunits efficiently mature in conformation. Replacing the N-terminal α helix of α7 with that of α1 prevented the expression of any mature α7 AChR, but replacing both the N-terminal α helix and the MIR loop of α7 with α1 increased expression of mature α7 AChRs on the cell surface 53-fold.3 Deleting or mutating the N-terminal α helix of many types of AChR subunits prevents assembly of mature AChRs.7 Mutations in or near the N-terminal α helix or MIR reduce expression of muscle AChRs causing myasthenic syndromes.8
Induction of EAMG
EAMG is usually induced by immunization of rats or mice with Torpedo electric organ AChR in adjuvant.2 Syngeneic muscle AChR can also be used. There is an acute phase of weakness 7–10 days after immunization resulting from the sudden appearance of autoantibodies to AChRs. It begins when low levels of antibody are first present in the serum and bound to AChRs in muscle, is mediated by binding of complement to these antibodies and release of C3 fragments of complement that attract macrophages that disrupt the postsynaptic membrane and ends when release of C3 is inhibited. There is a chronic phase of weakness starting about 30 days after immunization when high concentrations of autoantibodies develop in the serum and many of the AChRs in muscle are bound by antibodies. Then AChR loss is mediated by increased turnover due to endocytosis caused by crosslinking of the AChRs by antibody (termed antigenic modulation) and by focal lysis of the membrane due to complement cascades triggered by binding of antibodies. Complement mediated disruption of synapse structure and the relationship between pre- and postsynaptic components further impairs synaptic transmission. Chronic EAMG closely resembles MG.
Denatured AChR is profoundly less potent at inducing EAMG than is native AChR, although repeated immunization with denatured α1 subunits can induce mild EAMG.2,9 Passive immunization with MG patient autoantibodies or mAbs to the extracellular surface of muscle AChR can produce acute EAMG;2 mAbs to the MIR are very potent at passively transferring EAMG.
Induction of EAMG with MIR chimeras
Female Lewis rats were immunized once at day 0 in TiterMax adjuvant at the base of the tail with Torpedo AChR, human α1(1–32, 60–81)/AChBP MIR chimera, or wild-type AChBP.10
Immunization with Torpedo AChR at a dose of 11 µg produced acute EAMG in 4/6 and fatal chronic EAMG in 6/6 rats.10 The higher dose of 33 µg produced acute EAMG in 5/6 and chronic EAMG in 6/6, fatal in 5/6. Immunization with the MIR chimera produced acute EAMG in 3/6 at 11 µg, 5/6 at 33 µg, and 6/6 at 100 µg.10 These doses of the chimera produced chronic EAMG in 3/6, 6/6, and 6/6 and fatalities in 2/6, 2/6, and 5/6. Thus, the MIR chimera potently induced EAMG, though this one small part of a single muscle-like AChR subunit was not quite as potent as native Torpedo AChR. Immunization with wild-type AChBP was much less potent than the MIR chimera, producing no acute EAMG, and producing EAMG slowly and less severely.10 Chronic EAMG was 0/6 at 11 µg, 1/6 at 33 µg, and 3/6 at 100 µg with one fatality. The remarkable thing is that such a distantly related protein was able to induce EAMG at all. This implies that distantly related AChR-like proteins could, in principle, induce MG.
Properties of the antibodies induced
Immunization with Torpedo AChR produced large titers of antibody to itself after 120 days (750 nM at 11 µg and 2500 nM at 33 µg) but virtually none to the MIR chimera or to AChBP.10 It produced low levels to human muscle AChR (13 and 87 nM) and rat muscle AChR (1.4 and 13 nM).
Immunization with the MIR chimera produced substantial titers to the Torpedo AChR (550–750 nM).10 The MIR crossreacts relatively well between species, and the chimera has 5 α1 MIRs, whereas Torpedo AChR has 2 α1 MIRs, thus the chimera was more potent at making antibodies to Torpedo AChR than vice versa. The high intrinsic immunogenicity of the α1 MIR was demonstrated by the very high titers induced to the chimera (4,000–6,000 nM), as compared to AChBP (1,700–2,700 nM). The chimera also induced high titers to human muscle AChR (100–300 nM) and rat muscle AChR (18–55 nM). Immunization with AChBP produced very low titers to Torpedo AChR (≤ 67 nM), human muscle AChR (≤ 21 nM), or rat muscle AChR (≤ 2 nM).10 Moderate titers induced to itself (≤ 2,000nM) or the chimera (≤ 2,100 nM) emphasize the high intrinsic immunogenicity of the MIR.
All of the groups that developed EAMG developed similarly high titers to human AChR cytoplasmic domains, while those groups that did not develop EAMG developed no titers to cytoplasmic domains.10 Since AChBP and the chimera have no cytoplasmic domains, the antibodies to cytoplasmic antibodies produced after immunization with these must be in response to rat muscle AChRs exposed to the immune system through disruption of the postsynaptic membrane by the autoimmune response to the MIR or other surface epitopes on the AChBP. The high levels of antibodies to cytoplasmic domains indicate that endogenous AChR stimulates a robust autoimmune response once EAMG is initiated. Immunization with the whole extracellular domain of human α1 subunits expressed in bacteria was very weak at inducing EAMG because it lacks a native conformation (500 µg initiated EAMG in only 7/24 rats), but all those rats which did develop EAMG developed antibodies to cytoplasmic domains.11 Accounting for the fraction of α1 extracellular sequence used in the chimera and the dose necessary to produce EAMG in all of the immunized rats, the MIR/AChBP chimera is at least 200-fold more myasthenogenic than the bacteria-expressed α1 extracellular domain, providing an estimate of the benefit of expressing the α1 MIR in a nearly native conformation in an AChBP chimera.
Autoimmune stimulation by endogenous muscle AChR (as proven to occur by induction of antibodies to cytoplasmic domains by immunization with extracellular domains)10,11 may be important for sustaining the autoimmune response in EAMG after the response to the initiating immunogen wanes. Evidence for this is a continued increase in the concentration of serum antibodies to rat muscle AChR 30 days following the initial immunization with electric organ AChR, a time when the concentration of antibodies to electric organ AChRs decreases.12 Endogenous autoimmunization may also be important for sustaining MG. Specific immunosuppressive therapy of EAMG can be achieved by treatment for five weeks after the acute phase with a mixture of bacterially- expressed human muscle AChR subunit cytoplasmic domains.13 The mechanism of this therapy may involve antibody feedback, diversion, or other mechanisms. The target of this therapy may be the process of endogenous autostimulation of the response to muscle AChR, rather than suppression of the response to the initial immunogen.10
Antibodies from rats immunized with AChBP, the MIR chimera, or Torpedo AChR did not cross-react with human α3, α4, or α7 AChRs.10 Aplysia AChBP has 20% sequence identity with α1, 23% with α3, and 24% with α7. Much of the antibody to α1 AChRs induced by AChBP may be a result of autostimulation by muscle AChRs subsequent to very limited initial cross reaction. Lack of response to α3, α4, and α7 may reflect their lower antigenicity, immunogenicity, amount, concentration, or access to serum antibodies. Autonomic ganglia α3 AChRs can be the target of an antibody-mediated autoimmune attack, showing that they are accessible and vulnerable.14 Muscle α1 AChRs may be intrinsically more vulnerable as a result of intrinsic immunogenicity of the MIR, the large amounts of AChR per synapse, their density in the synapse, or other factors.
Conclusions
The α1 MIR is a potent immunogen that can efficiently induce EAMG and be a primary target of the autoimmune response. AChBP chimeras are excellent as immunogens and antigens for conformation-dependent AChR epitopes. Proteins distantly related to muscle AChRs, such as AChBP, can induce EAMG. Thus, such proteins from microbial or other sources could, in principle, trigger MG. An autoimmune response to epitopes on the extracellular surface of muscle AChR results in high levels of autoimmune response to AChR cytoplasmic domains when EAMG is induced. This “epitope spreading” indicates that the autoimmune response to AChRs in EAMG, and perhaps MG, is sustained by muscle AChRs. This autostimulation by muscle AChRs may be a target for specific immunosuppression of EAMG or MG.
Acknowledgments
This research was supported by grants from the NIH (NS11323 and NS052463) and the Muscular Dystrophy Association.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Tzartos S, Lindstrom J. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc. Natl. Acad. Sci. USA. 1980;77:755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindstrom J. Acetylcholine receptors and myasthenia. Muscle and Nerve. 2000;23:453–477. doi: 10.1002/(sici)1097-4598(200004)23:4<453::aid-mus3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Luo J, Taylor P, Losen M, de Baets M, Shelton G, Lindstrom J. Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. J. Neuroscience. 2009;29:13898–13908. doi: 10.1523/JNEUROSCI.2833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J. Mol. Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Brejc K, van Dyk W, Klassen R, Schuurmans M, vanderOost J, Smit A, Sixma T. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 6.Millar N. RIC-3: a nicotinic acetylcholine receptor chaperone. Brit. J. Pharmacol. 2008;153:S177–S183. doi: 10.1038/sj.bjp.0707661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo M, Mulet J, Aldea M, Gerber S, Sala S, Sala F, Criado M. Role of the N-terminal α helix in biogenesis of α7 nicotinic receptors. J. Neurochem. 2009;108:1399–1409. doi: 10.1111/j.1471-4159.2009.05924.x. [DOI] [PubMed] [Google Scholar]
- 8.Engel A, Shen X-M, Selcen D, Sine S. What we have learned from congenital myasthenic syndromes. J. Mol. Neuroscience. 2010;40:143–153. doi: 10.1007/s12031-009-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindstrom J, Einarson B, Merlie J. Immunization of rats with polypeptide chains from Torpedo acetylcholine receptor causes an autoimmune response to receptors in rat muscle. Proc. Natl. Acad. Sci. USA. 1978;75:769–773. doi: 10.1073/pnas.75.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo J, Lindstrom J. Myasthenogenicity of the main immunogenic region and endogenous muscle nicotinic acetylcholine receptors. Autoimmunity. 2012;45:245–252. doi: 10.3109/08916934.2011.622015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feferman T, Im S-H, Fuchs S, Souroujon M. Breakage of tolerance to hidden cytoplasmic epitopes of the acetylcholine receptor in experimental autoimmune myasthenia gravis. J. Neuroimmunol. 2003;140:153–158. doi: 10.1016/s0165-5728(03)00209-1. [DOI] [PubMed] [Google Scholar]
- 12.Lindstrom J, Lennon V, Seybold M, Whittingham S. Experimental autoimmune myasthenia gravis and myastheniagravis: biochemical and immunochemical aspects. Ann. N.Y. Acad. Sci. 1976;274:254–274. doi: 10.1111/j.1749-6632.1976.tb47691.x. [DOI] [PubMed] [Google Scholar]
- 13.Luo J, Kuryatov A, Lindstrom J. Specific immunotherapy of experimental myasthenia gravis by a novel mechanism. Ann. Neurology. 2010;67:441–451. doi: 10.1002/ana.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernino S, Lindstrom J, Hopkins S, Wang S, Low P. Characterization of ganglionic acetylcholine receptor autoantibodies. J. Neuroimmunology. 2008;197:63–69. doi: 10.1016/j.jneuroim.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen S, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontou M, Leonidas D, Vatzaki E, Tsantili P, Mamalaki A, Oikonomakos N, Acharya K, Tzartos S. The crystal structure of the Fab fragment of a rat monoclonal antibody against the main immunogenic region of the human muscle acetylcholine receptor. Eur. J. Biochem. 2000;267:2389–2397. doi: 10.1046/j.1432-1327.2000.01252.x. [DOI] [PubMed] [Google Scholar]
- 17.Delisanti C, Yao Y, Stroud J, Wang Z-Z, Chen L. Crystal structure of the extracellular domain of nAChR α1 bound to αbungarotoxin at 1.94 Å resolution. Nature Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]