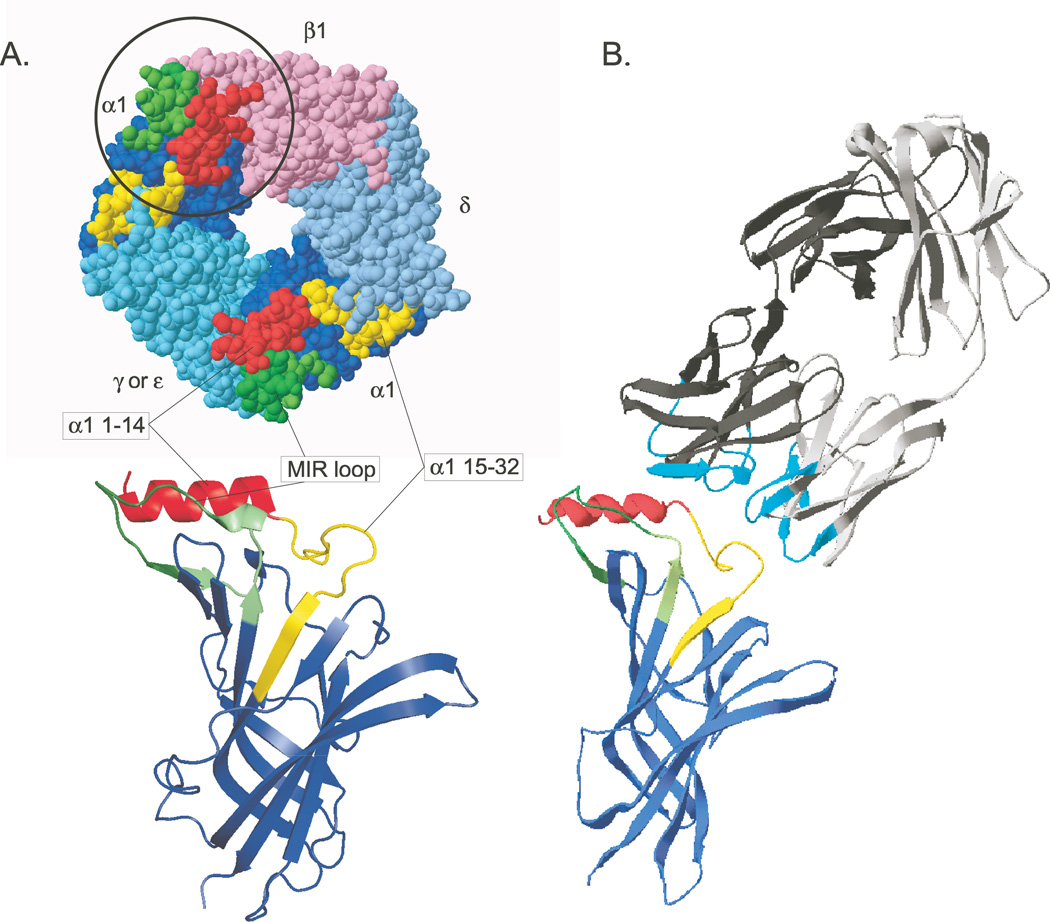
Figure 1.
A model of the MIR α1(1–32, 60–81)/AChBP chimera and its interaction with an antibody to the MIR is shown. (A) MIR components are highlighted on a top view of the crystal structure of an Aplysia AChBP subunit.15 Below, a front view ribbon diagram shows a single chimeric subunit. (B) The crystal structure of Fab 19216 is accompanied by the structure of the mouse α1 extracellular domain.17 Small differences in the sequences and conformation of the epitopes within the MIR profoundly influence the affinity with which antibodies are bound. The large size of bivalent IgG molecules with respect to the size of the MIR can result in competitive binding between different closely spaced epitopes within the MIR. The six hypervariable loops of the Fab, which form its antigen binding site are highlighted in cyan. This unusual mAb to the MIR does not appear to bind to the MIR loop per se, but competes for binding with mAbs, which do. The Fab is angled to suggest this, but not actually docked on the subunit model. This is part of Figure 1 from Luoet et al.3