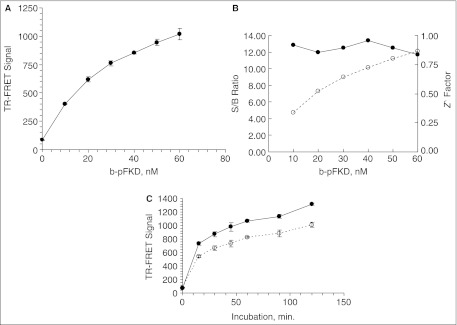
Fig. 4.
Development of Plk1 time-resolved fluorescence energy transfer (TR-FRET) assay in a 384-well format. (A) shows the signal dynamic range of the assay. The 400 nM of total mixture of b-FKD and b-pFKD with the indicated b-pFKD concentration was dispensed into 384-well plate in 10 µL of buffer A. After receiving 10 µL of TR-FRET reagent including Eu-p(S/T)F and SA-APC, the plate was further incubated for 45 min. Subsequently, relative fluorescence unit in 665 (RFU665) and 615 nm (RFU615) from APC and europium, respectively, with the excitation at 337 nm, were measured with Victor2™, and the TR-FRET signal was calculated (A). Error bars demonstrate standard deviation of the averages for n = 4. (B) S/B ratio (◯) and Z′ factor (●) at various b-pFKD concentrations. (C) Plk1 TR-FRET assay time-course experiment. See Materials and Methods for details. The 400 nM of b-FKD was incubated with 50 (◯) or 100 nM (●) Plk1ΔC in 20 µL for the indicated time. Error bars demonstrate standard deviation of the averages for n = 4.