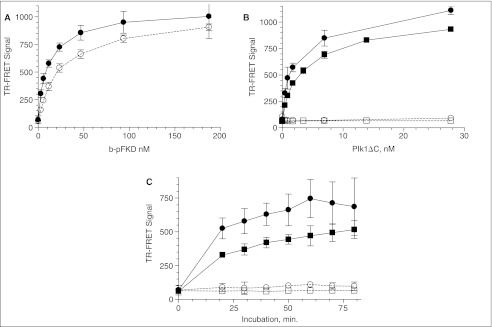
Fig. 5.
Miniaturization of Plk1 time-resolved fluorescence energy transfer (TR-FRET) assay in 1,536-well plate format. See Materials and Methods for details. The same batch of reagents was delivered to a 384-well plate with a Multidrop dispenser and a 1,536-well plate with a Flying Reagent Dispenser I. Y-axis shows the ratio of relative fluorescence unit (RFU) at 665 and 615 nm under the excitation of 337 nm measured with the Victor V. (A) Signal dynamic range. A 384- (◯) and 1,536 (●)-well plate received 250 nM mixture of b-FKD and b-pFKD with the indicated b-pFKD concentration in 10 µM ATP/buffer A, and EDTA/TR-FRET containing SA-APC and Eu-p(S/T)F. The volume of the peptide mixture and TR-FRET was 40 and 20 µL for 384-well plate and 4 and 2 µL for 1,536-well plate. The plate was incubated for 45 min before measuring RFUs with the Victor V. Error bars indicate standard deviation of the medians for n = 48 for a 384-well plate, and n = 192 for a 1,536-well plate. (B) Enzyme titration. The assay protocol is shown in Figure 1B and 1C. Indicated concentration of Plk1ΔC was incubated for 60 min at 25°ºC with 250 nM b-FKD peptide in the absence (□, ◯) or presence (■, ●) of 10 µM ATP for a 384- (■, □) and 1,536 (●, ◯)-well plate, respectively. Each plate received EDTA/TR-FRET, and was loaded onto the Victor2™ after 45 min. Error bars demonstrate standard deviation of the medians for n = 176 (●) and 16 (◯) for a 1,536-well plate, and n = 44 (■) and 4 (□) for a 384-well plate. (C) Time course. 9 nM Plk1ΔC was incubated for the indicated time with b-FKD peptide in the absence (□, ◯) or presence (■, ●) of ATP for a 384- (■, □) and 1,536 (●, ◯)-well plate, respectively. Error bars demonstrate standard deviation of the medians for n = 176 (●) and 16 (◯) for a 1,536-well plate, and n = 44 (■) and 4 (□) for a 384-well plate. Data at 0 min was from background wells that did not have Plk1ΔC.