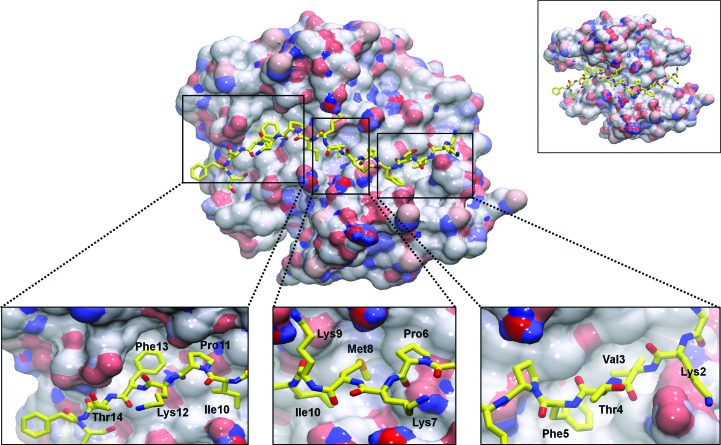
Figure 2.
AHNAK binds to various pockets on the surface of the S100A10–AnxA2 tetramer. The binding of AnxA2 to S100A10 increases the surface area of the largely hydrophobic surface formed across the dimeric interface of helix IV of S100A10 chains A and B by 363 Å2, allowing more interactions with AHNAK (yellow sticks). Removal of AnxA2 leaves the N- and C-terminal portions of AHNAK without a binding surface (upper right inset). Several hydrophobic residues of AHNAK insert themselves into hydrophobic pockets on the surface of S100A10–AnxA2, while the side chains of polar and charged residues mainly point towards the solvent (lower insets). The surface of S100A10–AnxA2 is colored by electrostatic potential, with red representing electronegative regions and blue representing electropositive regions.