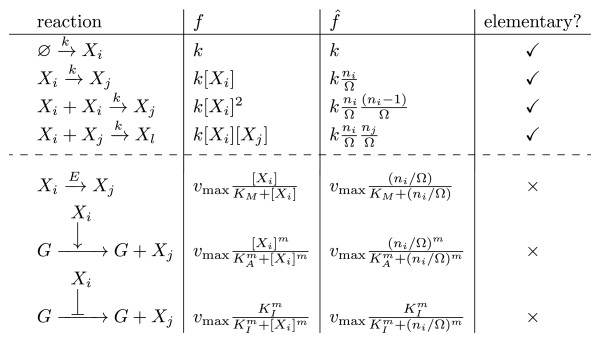
Figure 1.
Microscopic and macroscopic rate functions. The macroscopic rate function f and the microscopic rate function for various common types of chemical reaction steps. The former define the REs while the latter define the CME. The first four reactions are elementary, i.e., they are unimolecular or bimolecular reactions. The last three reactions are non-elementary, i.e., they can be decomposed into a number of simpler elementary reactions. These reactions represent (from top to bottom) the catalysis of a substrate by enzyme, up-regulation of a gene (G) by an activator and down-regulation of a gene by a repressor