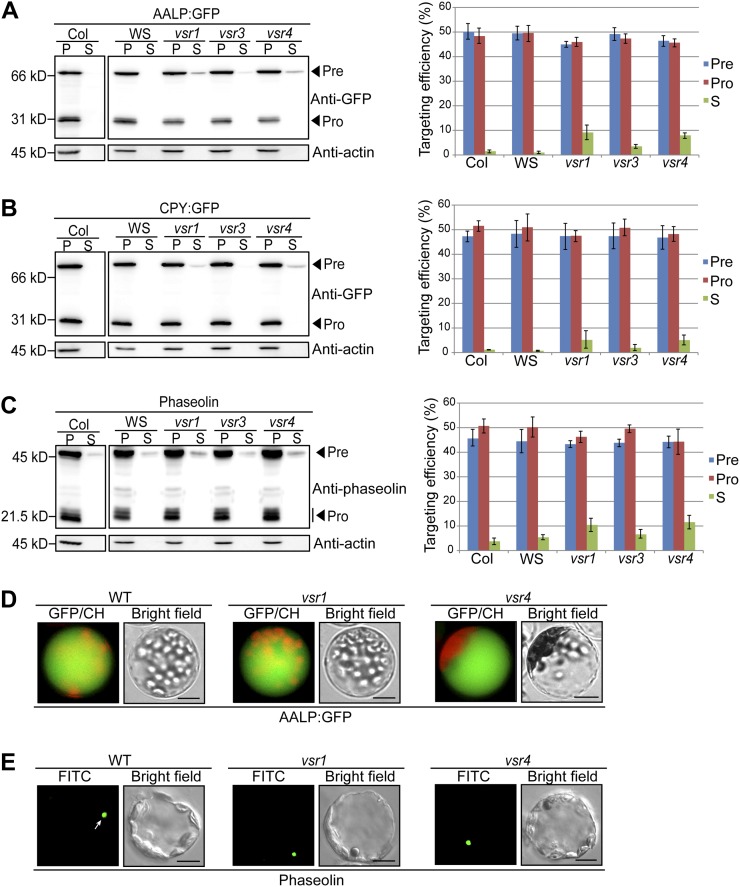
Figure 1.
Trafficking of vacuolar soluble cargoes is partially inhibited in vsr single mutants. A to C, Western-blot analysis of AALP:GFP, CPY:GFP, or phaseolin trafficking in vsr1, vsr3, and vsr4 mutants. Protoplasts from the wild type and vsr single mutants were transformed with AALP:GFP (A), CPY:GFP (B), or phaseolin (C). After 24 h of incubation, protein extracts were prepared from the protoplasts (P) and from the incubation medium (S). Twenty-five percent of protein extracts from the protoplasts and incubation medium were used for western-blot analysis. To measure the targeting efficiency, the amount of precursor (Pre), processed (Pro), and secreted (S) protein was quantified using multigauge software and expressed as a value relative to the amount of total protein (proteins in protoplasts and incubation medium). Actin detected with antiactin antibody was used as a control for protein leakage from protoplasts and as a loading control. Three independent transformation experiments were performed to obtain an average targeting efficiency with sd. Error bars indicate sd (n = 3). WS, Wassilewskija wild type. D and E, Subcellular localization of AALP:GFP (D) and phaseolin (E) in vsr1 and vsr4 mutant protoplasts. Protoplasts derived from wild-type and vsr1 and vsr4 mutant plants were transformed with AALP:GFP and phaseolin, respectively. At 24 h after transformation, localization of these cargo proteins was examined under the fluorescent microscope. The fluorescent signal of AALP:GFP and CPY:GFP was observed directly. Localization of phaseolin was examined by laser-scanning confocal microscopy. Transformed protoplasts were immunostained with an antiphaseolin antibody followed by an FITC-labeled secondary antibody. Arrow indicates the localization of phaseolin. CH, Chloroplast autofluorescence; WT, Col-0 wild type. Bars = 20 μm.