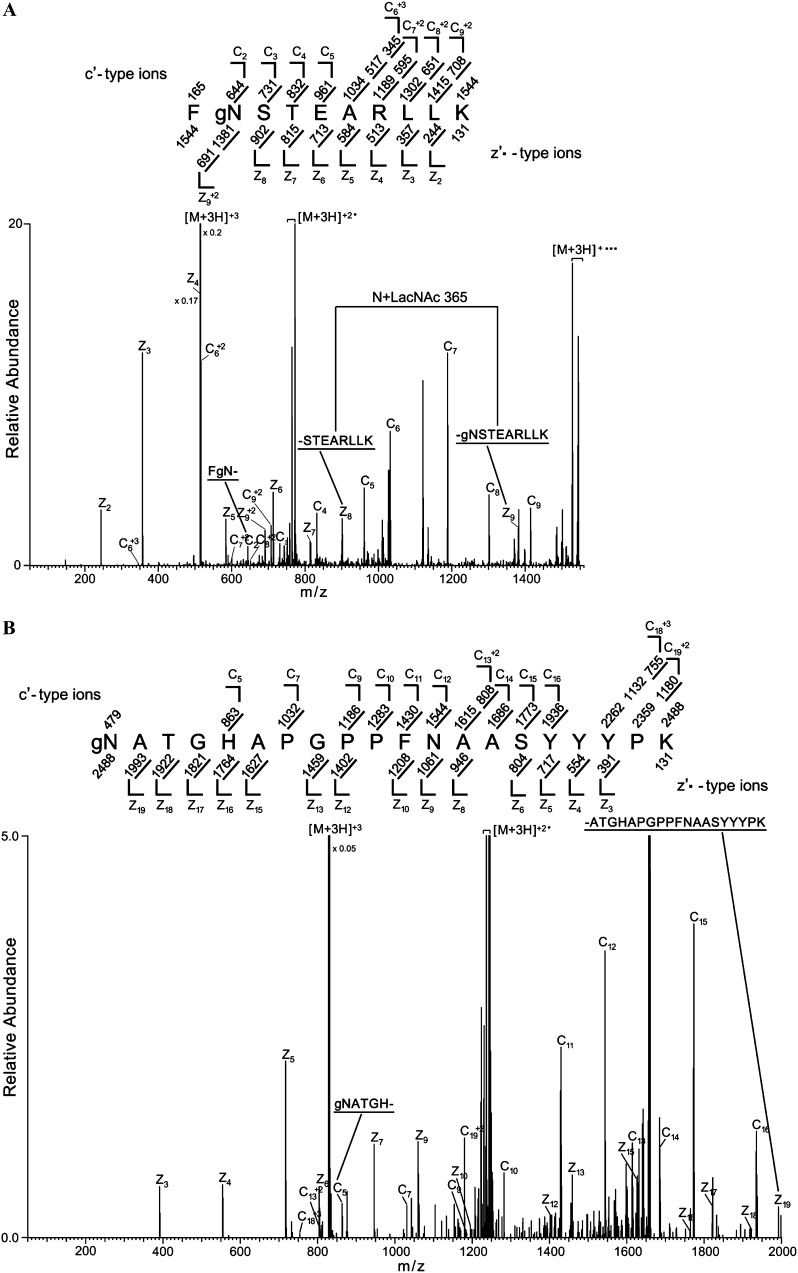
Figure 3.
TGG1 and TGG2 are N-GlcNAc modified. Proteins from the fucta/fuctb/xylt triple mutant were digested with Lys-C (or trypsin) and treated with PNGase F. Glycans with terminal GlcNAc were capped with Gal, and GlcNAc-modified peptides were enriched by RCA I lectin affinity chromatography (Supplemental Fig. S2). The enriched peptides were then analyzed by mass spectrometry. A, ETD MS/MS spectrum recorded on [M+3H]+3 ions (m/z 515.2663) from the LacNAc (365.1322)-modified TGG2 peptide FgNSTEARLLK. B, [M+3H]+3 ions (m/z 830.0481) corresponding to the LacNAc-modified TGG1 peptide gNATGHAPGPPFNAASYYYPK. Predicted c′- and z′·-type ions are listed above and below the peptide sequence, respectively. Singly and doubly charged fragment ions are listed as monoisotopic masses. Ions observed and labeled in the spectrum are underlined. Ions corresponding to charge-reduced species and those resulting from neutral losses are bracketed. Modified residues are preceded by “g” to signify modification by a single LacNAc moiety.