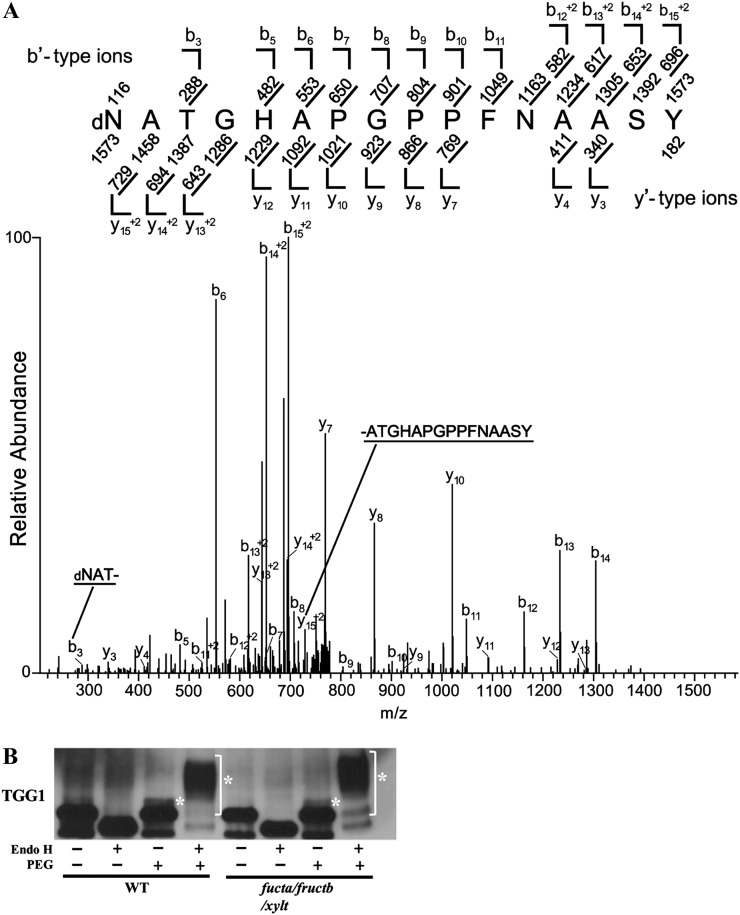
Figure 5.
High-Man-type glycans occur at multiple sites on TGG1, including N379, the site of N-GlcNAc modification. A, TGG1 was enriched by Con A-Sepharose affinity chromatography, treated with PNGase F, and further purified by 2D PAGE. TGG1 was cut out from a 2D gel, digested with trypsin, and analyzed by MS/MS. The CID MS/MS spectrum was recorded on [M+2H]+2 ions (m/z 786.8574) corresponding to the peptide dNATGHAPGPPFNAASYYYPK deamidated at Asn (N379). Predicted b′- and y′-type ions are listed above and below the peptide sequence, respectively. Singly and doubly charged fragment ions are listed as monoisotopic masses. Ions observed and labeled in the spectrum are underlined. The modified residue is preceded by “d” to signify deamidated Asn. B, An immunoblot showing that extensive PEGylation of TGG1 occurs after treatment with Endo H. Asterisks indicate the PEGylated proteins.