Scheme 2.
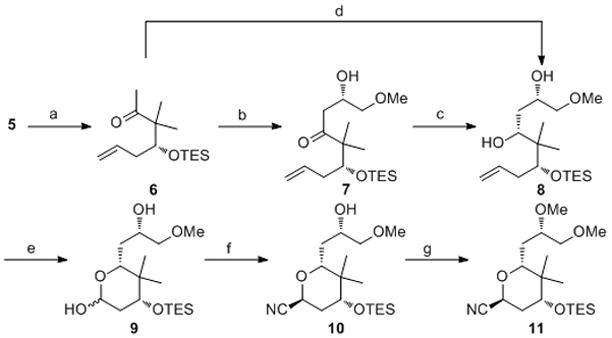
Synthesis of the nitrile component. Reagents and conditions: a) TESCl, imidazole, CH2Cl2, 100%; b) (+)-DIP-Cl, Et3N, MeOCH2CHO, Et2O, −78 °C, 88%, dr = 15:1; c) NaBH4, Et2BOMe, MeOH, THF, −40 °C, 95%; d) (+)-DIP-Cl, Et3N, Et2O, −78 °C, then LiBH4, −40 °C, 80%; e) O3, CH2Cl2, −78 °C, then Ph3P, 95%; f) TMSCN, BiBr3, CH3CN, 0 °C, then BF3•OEt2, −40 °C, 63%; g) MeOTf, 2,6-tBu2Py, CH2Cl2, 86%. DIP-Cl = B-chlorodiisopinocam-phenylborane, TMSCN = trimethylsilyl cyanide, MeOTf = methyl trifluoromethanesulfonate, 2,6-tBu2Py = 2,6-di-tert-butylpyridine.
