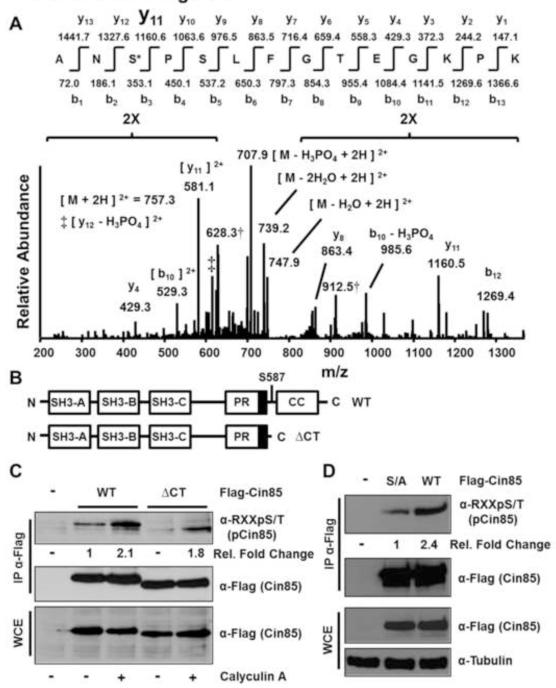
Fig. 2.
Serine 587 is the primary Cin85 phosphorylation site recognized by the anti-phospho-RXXS/T motif antibody from growing HEK 293 cells. (A) Low energy collision-induced dissociation (CID) MS/MS spectrum identified from peptides extracted following in-gel tryptic digestion of the Flag-Cin85 band shown in Fig. 1D. Compare this spectrum to the spectrum from the unphosphorylated peptide shown in Fig. S1. (B) Schematic of the domain structure of Cin85. The three Src homology 3 (SH3) domains are depicted as well as a proline-rich (PR) region. The dark box in the PR region indicates the CapZ binding region. CC indicates the coiled-coil domain. (C) Cells were transfected with the indicated constructs and experiments were conducted as described in the legend to Fig. 1B. Fold changes in phospho-Cin85 levels relative to Flag-Cin85 levels and sample type are indicated. (D) Cells were transfected with the indicated constructs (S/A indicates Ser587Ala) and experiments were conducted as described in Fig. 1A. Fold changes in phospho-Cin85 levels relative to Flag-Cin85 levels and sample type are indicated.