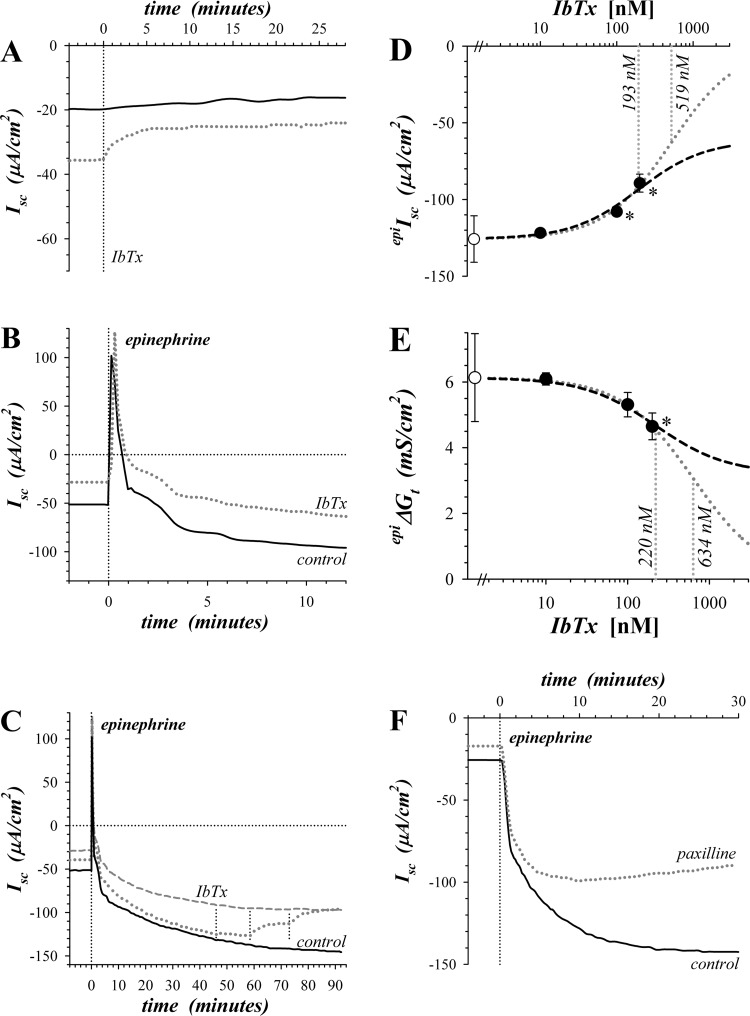
Fig. 1.
Sensitivity of epinephrine activated secretion to KCa1.1 blockers. Isolated mucosae were stimulated by epinephrine addition to the serosal solution from the standard basal condition with electrogenic secretion monitored by short-circuit current (Isc) (see methods). A: Isc was measured in adjacent mucosae during basal conditions with addition of IbTx (300 nM) to the mucosal solution of one (dotted line). B: adjacent mucosae were stimulated by epinephrine (5 μM) with one pretreated by mucosally added IbTx (200 nM) 60 min prior to epinephrine (dotted line). C: a third mucosa adjacent to those shown in panel B was treated with three successive concentrations of IbTx (10, 100, and 200 nM) beginning after epinephrine stimulation (dotted line), control (solid line), and IbTx pretreatment (dashed line). D and E: responses of epinephrine-activated Isc (epiIsc) and transepithelial conductance (epiΔGt) to 3 concentrations of IbTx were measured with an adjacent mucosa as time control. Control epiIsc and epiΔGt were −125.8 ± 15.2 μA/cm2 and +6.13 ± 1.34 mS/cm2 (○, n = 3), respectively. Fractional inhibition by IbTx was corrected for any time-dependent changes, averaged, and scaled to the control epiIsc or epiΔGt (●). Values significantly different from control (P < 0.05) are indicated by an asterisk (*). A fit of Henri-Michaelis-Menten kinetics with a single binding site was made to the concentration dependence from each mucosa and the average (n = 3) shown as a dashed line: epiIsc, IbTxIC50 = 193 ± 41 nM, maximal fractional inhibition = 0.513 ± 0.121; epiΔGt, IbTxIC50 = 220 ± 77 nM, maximal fractional inhibition = 0.476 ± 0.040. An additional fit was made assuming that IbTx could inhibit epiIsc or epiΔGt completely (dotted line). F: adjacent mucosae were stimulated by epinephrine (1.0 μM) with one pretreated by paxilline (1.0 μM) addition to both mucosal and serosal solutions 35 min prior to epinephrine (dotted line).