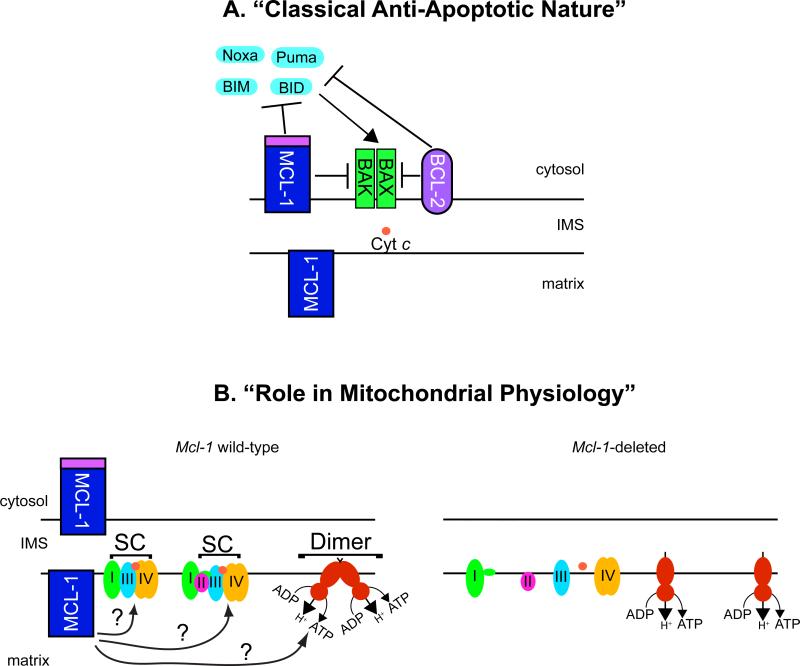
Figure 2. Model for MCL-1's Potential Functions at the Mitochondria.
MCL-1 possesses multiple functions at the mitochondria. (a) On the outer mitochondrial membrane (OMM), MCL-1 functions like other anti-apoptotic BCL-2 family members where it acts to prevent the activation of BAX and BAK to prevent cell death. MCL-1 can directly bind BH3-only family members, such as BIM, sequestering them away from the pro-apoptotic effectors BAX or BAK. Alternatively, MCL-1 may directly bind BAX and BAK and maintain them in an inactive conformation. (b) During mitochondrial importation, the full-length MCL-1 is proteolytically truncated on its amino-terminus. The truncated, matrix localized MCL-1 resides within the inner mitochondrial membrane where it functions to maintain mitochondrial cristae ultrastructure and promotes the assembly of the electron transport chain complexes into higher-order assemblies known as supercomplexes (SC). The assembly into supercomplexes has been shown to facilitate electron transport efficiency and reduce the production of deleterious reactive oxygen species. Additionally, matrix-localized MCL-1 facilitates the assembly of the higher-order assembly of the ATP synthase complexes into dimers and oligomers. Proper assembly of oligomeric ATP synthase has been implicated in being an important determinate of inner membrane cristae structure. Genetic ablation of Mcl-1 results in defects in both supercomplex and ATP synthase oligomer assembly. Whether MCL-1 acts directly or indirectly to facilitate these macromolecular assemblies of the electron transport supercomplexes or ATP synthase oligomers is still unclear.