Abstract
The fourth subunit of the epithelial sodium channel, termed delta subunit (δ ENaC), was cloned in human and monkey. Increasing evidence shows that this unique subunit and its splice variants exhibit biophysical and pharmacological properties that are divergent from those of α ENaC channels. The widespread distribution of epithelial sodium channels in both epithelial and nonepithelial tissues implies a range of physiological functions. The altered expression of SCNN1D is associated with numerous pathological conditions. Genetic studies link SCNN1D deficiency with rare genetic diseases with developmental and functional disorders in the brain, heart, and respiratory systems. Here, we review the progress of research on δ ENaC in genomics, biophysics, proteomics, physiology, pharmacology, and clinical medicine.
Keywords: non-voltage-dependent sodium channels, genetics, pharmacology, biophysics, physiological regulation, diseases
four subunits of the epithelial sodium channel (ENaC α, β, γ, and δ subunits) have been cloned in mammals to date (26, 90, 134). The widely accepted concept is that the functional ENaC channels must be composed of at least one α or α-like subunit (δ and δ-like ε ENaC from Xenopus laevis). The β and γ subunits are both required to amplify channel activity up to two orders of magnitude. In contrast to α and β ENaC, δ ENaC is widespread and expressed in both nonepithelial (brain, heart, ganglion, placenta, blood, etc.) and epithelial cells (trachea, kidney, pancreas, liver, stomach, etc.), similar to the expression profile of γ ENaC. However, the γ ENaC gene (SCNN1G) shares the cytogenetic band (16p12.2) of human chromosome 16 with β ENaC but is separated from the δ ENaC gene (SCNN1D). There are a number of classic reviews about the native and heterologous (αβγ) epithelial sodium channels before and after the initial cloning of ENaC in 1993 (4, 11, 42, 52, 66, 67, 78, 81, 94, 95, 105, 108, 118, 123, 129). Until very recently, only one review was published focusing on δ ENaC (54). We therefore summarize current understanding of the δ ENaC in this review.
Genomics of δ ENaC (SCNN1D)
Chromosome assignment.
The first cDNA sequence of SCNN1D (dNaCh or ENaCdelta, Homo sapiens) was published in 1995 (134). So far, the sequence of SCNN1D is defined by 120 GenBank accessions from 108 cDNA clones from testis, brain, lung, pancreas, and other tissues. It was assigned to human chromosome 1p36.3-p36.2 with a sequence ID, NC_000001.10 (136). It covers 18.03 kb, from 1209383 to 1227414 (NCBI 37, August 2010), on the direct strand. To date, four homologenes and 31 gene orthologs have been deposited to NCBI and Ensembl databases, respectively, including Pan troglodytes (located on chromosome 1, NC_006468.3), Canis lupus familiaris (chromosome 5, NC_006587.2), Bos taurus (chromosome 6, NC_007314.3), and Gallus gallus (chromosome 21, NC_006108.2). By aligning their sequences (Fig. 1A), up to 99.1% identity among them was found. Most of them are from vertebrate mammals except the chicken, the frog, the green anole, and the finch. Unfortunately, neither SCNN1D DNA nor RNA has been identified in the mouse, the most popular animal model for manipulating the other three ENaC genes (SCNN1A, SCNN1B, and SCNN1G). Human SCNN1D is located between genes UBE2J2 and ACAP3 on chromosome 1p36 (130). In contrast, UBE2J2 adjacent to the ACAP3 gene has been found in mouse chromosome 4 (Fig. 1B). At present, genetic analysis shows that SCNN1D is a pseudogene in mice (54). Three genes, namely MAPK8IP3 (mitogen-activated protein kinase 8 interacting protein 3), STK11 (serine/threonine kinase 11), and C2orf55 (chromosome 2 open reading frame 55), are coexpressed with the SCNN1D.
Fig. 1.
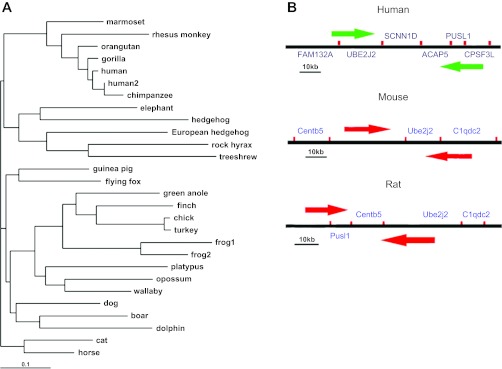
Phylogenetic and genomic analysis of SCNN1D. A: phylogenetic tree of SCNN1D across species. Software TreeView version 1.6.6 was used to construct phylogenetic tree. B: comparison of SCNN1D-bearing chromosome in human with murine chromosomes only containing adjacent genes.
Tissue expression profile.
The human SCNN1D gene contains 33 distinct introns (28 gt-ag, 2 gc-ag, 3 others) (130). Transcription of SCNN1D gene is predicted to produce 15 different mRNAs (14 alternatively spliced variants and 1 unspliced form) (130). To date, two transcriptions have been identified by several groups (53, 147, 155). There are five probable alternative promoters, two nonoverlapping alternative last exons, and four validated alternative polyadenylation sites (130). The mRNAs appear to differ in the truncation of their 5′ and 3′ ends, presence or absence of eight cassette exons, overlapping exons with different boundaries, and splicing vs. retention of nine introns (130).
The tissue expression profile of SCNN1D has originally been examined by Waldmann et al. (134). This report was followed by two extensive studies using dot-blot and gene chip microarray (124, 148). These data are summarized in Table 1. SCNN1D is predominantly expressed in heart, liver, brain, and lung (including trachea) as seen by all three studies, and pancreas, skeletal muscle, and blood leukocytes by two reports. Clearly, transcription levels of δ ENaC in both epithelial and nonepithelial tissues are ranked top ten (124, 134, 148). According to the largest human gene expression database, BioGPS, there appear two diverse expression patterns of human ENaC (Table 1). SCNN1A and SCNN1B are predominantly expressed in the lung and the kidney, as shown in Fig. 2. For example, the expression level in the lung was ∼240- and 50-fold the median value of all tissues analyzed, respectively, for SCNN1A and SCNN1B. By contrast, SCNN1G and SCNN1D are widely expressed in both epithelial and nonepithelial tissues.
Table 1.
Top 10 human tissues with robust δ ENaC (SCNN1D) expression profiles
| Gene (Median) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| SCNN1Da | Testis | Ovary | Pancreas | Brain | Heart | Thymus | Skeletal muscle | Liver | Lung | Blood leukocytes |
| SCNN1Db | Heart | Kidney | Liver | Brain | Pancreas, Fetal Kidney | Placenta, Stomach | Trachea, Fetal heart, Bone marrow | Adrenal gland | Transverse colon, Duodenum, Jejunum | Spleen, Fetal lung, Fetal brain |
| SCNN1D (4.3)c | Liver (7.1) | Heart (7.1) | Thyroid (6.5) | CD34+ (6.5) | Skeletal muscle (6.5) | Prefront cortex (6.15) | Retina, Prostate, Ganglionf (6.1) | Pituitary (5.9) | Smooth muscle (5.65) | Lung, Whole blood (5.5) |
| SCNN1Ad (5.3) | Lung (733) | Kidney (492) | Thyroid (444) | Trachea (347) | Colon (290) | Prostate (245) | Salivary gland (135) | Tongue (108) | Pancreatic islet (102) | Retina (71) |
| SCNN1Be (4) | Lung (150) | Tongue (30) | Placenta (26) | Skin (10) | Kidney (9) | Thyroid (8) | Salivary gland (6) | Globus pallidus (5.4) | Bone marrow (5.3) | Heart, Liver, Lymphocyte, DRG (5) |
| SCNN1G (5.1) | Liver (4.5) | Heart (4.35) | Prefron-tal (4.35) | CD34+ (4.3) | Thyroid (4.3) | Skeletal muscle (4.15) | Prostate (4.1) | Retina (4.18) | Pituitary (4.0) | Smooth muscle (3.9) |
Normalized expression levels are in parentheses. ENaC, epithelial sodium channel. aData are adapted from Waldmann et al. (134): Northern blot with mRNA samples for 16 types of tissues (5.5 kDa). bData are adapted from Yamamura et al. (148): Dot-blot with 73 human samples for 73 types of tissue/organ. cAdapted from BioGPS (http://biogps.org/#goto=welcome) (142). The microarray database “GeneAtlas U133A, gcmas” was produced by an Affymetrix U133A array with data processed by the gcrma algorithm. Data were statistically analyzed for 176 human samples for 84 types of tissue/organs (124). dCCIB rank: 1 kidney, 2 conjunctiva, 3 trachea/lung, 4 stomach/esophagus, 5 thyroid /ES cell, 6 salivary gland, 7 breast, 8 prostate, 9 tongue, 10 skin. eCCIB rank: 1 esophagus, 2 tongue, 3 lung, 4 kidney, 5 placenta, 6 skin, 7 stomach, 8 salivary gland, 9 colon, 10 globus pallidus/Treg/superior cervical ganglion. fSuperior cervical ganglion (6.35) and trigeminal ganglion (5.85).
Fig. 2.
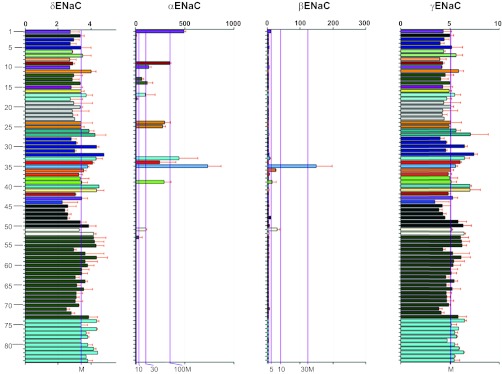
Gene expression profile of SCNN1A, SCNN1B, SCNN1G, and SCNN1D. Expression levels are computed from the “GeneAtlas U133A, gcmas” dataset, an atlas of tissue expression (GeneAtlas) on an Affymetrix U133A array, using the gcrma algorithm to process the data. The y-axes on these graphs represent normalized, background-subtracted, and summarized (probes to probeset) intensity of each probe set: 1 kidney, 2 tonsil, 3 lymph node, 4 thymus, 5 bone marrow, 6 adrenal gland, 7 adrenal cortex, 8 olfactory bulb, 9 trachea, 10 salivary gland, 11 pituitary, 12 fetal liver, 13 fetal lung, 14 fetal thyroid, 15 uterus, 16 adipocyte, 17 pancreatic islet, 18 pancreas, 19 testis seminiferous tubule, 20 testis Leydig cell, 21 testis interstitial, 22 testis germ cell, 23 testis, 24 colorectal adenocarcinoma, 25 bronchial epithelial cells, 26 smooth muscle, 27 cardiac myocytes, 28 leukemia lymphoblastic (MOLT-4), 29 leukemia chronic myelogenous K-562, 30 lymphoma Burkitts (Daudi), 31 leukemia promyelocytic HL-60, 32 leukemia Burkitts (Raji), 33 thyroid, 34 prostate, 35 lung, 36 placenta, 37 CD71+ early erythroid, 38 small intestine, 39 colon, 40 liver, 41 heart, 42 uterus corpus, 43 appendix, 44 ovary, 45 dorsal root ganglion, 46 ciliary ganglion, 47 atrioventricular node, 48 skin, 49 trigeminal ganglion, 50 superior cervical ganglion, 51 tongue, 52 skeletal muscle, 53 retina, 54 pineal night, 55 pineal day, 56 whole brain, 57 amygdala, 58 prefrontal cortex, 59 spinal cord, 60 hypothalamus, 61 fetal brain, 62 thalamus, 63 caudate nucleus, 64 parietal lobe, 65 medulla oblongata, 66 cingulate cortex, 67 occipital lobe, 68 temporal lobe, 69 subthalamic nucleus, 70 pons, 71 globus pallidus, 72 cerebellum, 73 cerebellum peduncles, 74 CD34+, 75 CD105+ endothelial, 76 721 B lymphoblasts, 77 CD19+ B cells (neg._sel.), 78 BDCA4+ dendritic cells, 79 CD8+ T cells, 80 CD4+ T cells, 81 CD56+ NK cells, 82 CD33+ myeloid, 83 CD14+ monocytes, 84 whole blood. The bottom x-axes show the median (M) and the fold median level, as indicated by purple lines, and the top x-axes show the relative expression level with light gray lines; 176 samples for 84 types of tissues were statistically analyzed. Data are adapted from BioGPS with permission (www.BioGPS.org).
Interestingly, the expression level of all ENaC subunits in fetal lungs is much lower than in adult lungs, in particular the α and β subunits (Table 2). The increment in ENaC expression in adulthood is seen in both reabsorptive epithelium and nonepithelial tissue (e.g., thyroid). Similarly, dot-blot showed that the transcription level of δ ENaC in fetal kidney, heart, and brain is much less than corresponding adult tissues (Table 1) (148). These data indicate that ENaC expression occurs in a development-dependent manner.
Table 2.
Normalized expression levels of ENaC subunits in human respiratory epithelium (trachea, bronchi, and lung)
| α ENaC | β ENaC | γ ENaC | δ ENaC | |
|---|---|---|---|---|
| Trachea | 133 | 3.3 | 4.2 | 2.9 |
| Bronchial epithelial cells | 104.8 | 3.9 | 4.9 | 3.4 |
| Fetal lung | 33.9 | 3.6 | 4.1 | 2.9 |
| Adult lung | 257 | 150 | 5.6 | 3.8 |
| Kidney | 184.8 | 9.9 | 4.3 | 2.8 |
| Fetal thyroid | 56.4 | 3.9 | 4.9 | 3.3 |
| Adult thyroid | 170.4 | 4.9 | 6.5 | 4.3 |
The kidney and the thyroid are controls of epithelial and nonepithelial tissues, respectively. Data are adapted from BioGPS with permission (http://biogps.org/#goto=welcome).
In addition to whole-organ/tissue level expression, δ ENaC mRNA was also found in primary and immortalized human cells. Recently, δ ENaC was identified in human nasal epithelial cells (8), human glioblastoma (14), immortalized human respiratory epithelial cells (A549, H441, Calu-3, 16HBE14o−), human pancreatic epithelial cells (CFPAC), human colonic cells (Caco-2), human pleural mesothelial cells (M9K), human primary alveolar type II cells, human primary pleural mesothelial cells, human esophageal cells, human melanoma cells (G361), and human lung tissues (75, 99, 101, 111, 143, 145). δ ENaC-like transcripts were also found in rabbit lung and retinal tissues by PCR using primers designed to detect the human δ ENaC sequence (20, 75). An RNA product of similar size to the human mRNA was also amplified in murine cells and tissues (63, 75). Given that the δ ENaC gene has not been identified in mouse and rabbit, the PCR products reported are of uncertain origin. In addition, its counterpart in Xenopus laevis (ε xENaC) was predominantly expressed in kidney and urinary bladder, but faintly expressed in skeletal muscle and brain (7).
Intraorgan distribution.
1) Lung: Two splice variants of δ ENaC were analyzed in human alveolar epithelial cells. In both alveolar type I and II cells, δ2 ENaC is expressed at a lower level than δ1 ENaC (155). In some cases, δ1 and δ2 were present in the same alveolar cells. In addition, δ1 ENaC was also present in pulmonary leukocytes, in which other ENaC subunits and amiloride-sensitive channels have been detected (24). 2) Esophagus: The expression pattern of δ ENaC was examined in human esophagus by in situ hybridization (143). Abundant mRNA expression was detected throughout the esophageal surface layer, consisting largely of stratified squamous epithelium. Trace levels of expression were observed in submucosal glands. δ ENaC on the esophageal surface may detect the refluxed gastric acid and convert and transduce the signal via terminal neuroreceptors. 3) Eye: Recently, the copy number of ENaC subunits in human eye tissues was measured by quantitative real-time RT-PCR (86). Expression of δ ENaC at the mRNA level is similar to β and/or γ subunits in human lens epithelium, iris, retina, ciliary processes, and choroid. Moreover, no significant difference in expression level was found between δ and α subunits in iris and retina (86). 4) Brain: Distribution of δ ENaC (δ1) and a splice variant (δ2) were analyzed in human brain by dot-blot and in situ hybridization (53, 148). δ ENaC was almost evenly expressed in 16 areas of adult brain except frontal lobe, medulla oblongata, accumbens nucleus, thalamus, and corpus callosum (148). In contrast, spinal cord showed a very weak expression level. Furthermore, δ ENaC-expressing cells included pyramidal neurons in layers II to VI and the frontal and temporal cortices (53). Most pyramidal neurons express either δ1 or δ2 ENaC. Their distribution pattern varies from one cortical region to another. Furthermore, δ1 transcript levels were 2.5-fold that of δ2 ENaC in human cerebral cortex, as analyzed by quantitative PCR (138). In addition, the cell-specific distribution of δ ENaC in tongue can be found in the following sections.
Translation of SCNN1D.
The encoded proteins of the four SCNN1D homologenes show a high identity to human δ1 (99.1, 67.9, 67.4, and 47.7%, respectively vs. P. troglodytes, C. lupus, B. Taurus, and G. gallus). The human EST database shows two alternatively spliced exons and one alternatively spliced intron: 1) exon 3 is occasionally spliced out; 2) exon 6 is more frequently spliced out; 3) intron 10 retention has been found in several EST clones. For SCNN1D splice variants, exon 6 deletion causes a frame shift and premature termination of translation. An alternative translation with a different reading frame can result in a similar product with a different NH2-terminus (53). Intron 10 retention also results in a frame shift and premature termination halfway through the transcript (position 468). By BLASTing the NCBI protein database, nine hits were obtained for human δ ENaC proteins with potential channel function (Fig. 3), including the original δ1 (CAI23262.1) and recently described δ2 (CAI23261.1). These splice variants showed greatest variability in their intracellular amino-terminal tail (Fig. 3A) and can be classified into two groups. The δ1 ENaC amino-terminal tail has 638 amino acid residues (aa) whereas group one consists of five splice variants with extended amino-terminal tails: 771 aa (EAW56254.1), 802 aa (NP001123885.2), 704 aa (CAI23261.1, δ2 ENaC), 669 aa (EAW56251.1), and 721 aa (EAW56252.1). Group two consists of three truncated variants [CAI23263.1 (307 aa), CAI23260.1 (460 aa), and AAI25076.1 (378 aa)] with shorter NH2-terminal tails and additional missing regions in the extracellular loop, transmembrane domains, and carboxy-terminal tails. The full-length protein apparently has 802 aa residues. The group one variants with lengths between 600 and 800 aa residues seem to be spliced with a local frame shift (one exon deletion causes frame shift, and a second exon deletion shifts the frame back). Given the critical roles of these missing domains in exocytosis and channel pore formation, the group two variants may not assemble as functional channels in the plasma membrane and may be electrically undetectable by electrophysiological approaches. In addition, few short peptides have been predicted by NCBI AceView (130).
Fig. 3.
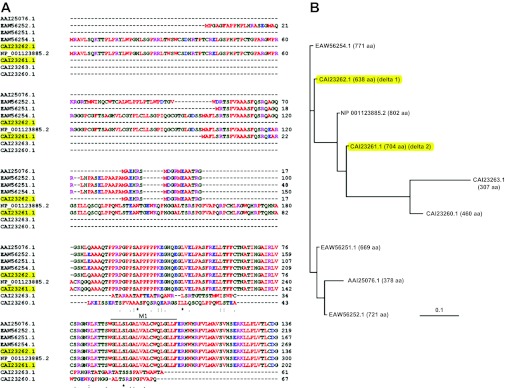
Translated proteins of SCNN1D. A: alignment of the amino acid sequences of amino-terminal tails; δ1 (CAI23262.1) and δ2 (CAI23261.1) are highlighted. M1, first transmembrane domain. B: polymorphic tree of SCNN1D proteins. The predicted translations of δ ENaC proteins are aligned with the NCBI database IDs. The total numbers of amino acids are indicated in parentheses; δ1 and δ2 proteins are highlighted.
Human δ ENaC expressed in nonpolarized COS-7 cells appears as two specific bands at 85 and 95 kDa when probed with anti-HA (flag) antibody by Western blot assay (28, 58). The 95-kDa band shifted down by 5 kDa (to 90 kDa) by pretreatment of δ ENaC precipitate with N-glycosidase F, suggesting that the size of glycosylated δ ENaC is ∼95 kDa. In contrast, δ ENaC appears as two prominent bands at 86 and 75 kDa representing the glycosylated and nonglycosylated forms, respectively, when expressed in Xenopus oocytes (58). Moreover, δ ENaC protein density at the cell surface, as revealed by immunofluorescence assay, was almost the same as that for α ENaC in human primary nasal epithelial cells. Western blot assay showed that δ ENaC was recognized by a specific antibody at 100 kDa (8). In addition, δ ENaC proteins (85 or 100 kDa) have been reported in confluent human lung epithelial cells (H441, Calu-3, 16HBE14o−), human primary pleural mesothelial cells, M9K cells, human lung tissues, and human melanoma cells by immunofluorescence and immunoblotting assays (75, 99, 130, 145, 150). As predicted, δ ENaC may interact with other ENaC subunits, COMMD1, syntaxins, Nedd4, and ERK1.
Biophysical and Pharmacological Features
Macroscopic activity of heteromultimeric channels expressed in Xenopus oocytes. δ ENaC alone formed a monomultimeric channel in Xenopus oocytes with a whole-cell current in the range of −50 nA (71, 148). When δ ENac was coexpressed with β and γ subunits, the amplitude of the macroscopic currents increased by more than two orders of magnitude. One group reported that the amiloride-sensitive whole-cell currents were ∼11-fold higher in oocytes expressing δβγ than those expressing αβγ channels (58). A recently cloned δ2 ENaC recorded greater whole-cell currents when coexpressed with βγ subunits in oocytes (155). In addition, less than or equal to the current amplitude of δ1βγ in oocytes overexpressing δ1βγ clone was found (138, 147). The divergent observations may result from variable quality in oocytes, cRNA, and other experimental conditions. Without specific pharmacological and molecular approaches, the functions of native δ1 and δ2 ENaC channels have not been studied. The apparent half-saturation concentration (Km) of δ2βγ for external Na+ ions was 18% greater than that of δ1βγ, indicating a lower Na+ affinity (155). The channels containing δ subunit alone share biophysical and pharmacological features with channels comprised of δ, β, and γ subunits as described below.
Cation selectivity.
Increased permeability of Na+ over Li+ ions (ILi/INa = 0.6) through δβγ channels is one of three features that diverge from αβγ channels (ILi/INa = 2) (71, 72, 134). Furthermore, channel permeability to both monovalent and divalent cations was examined. The PX-to-PNa ratios of Na+/Li+/K+/Cs+/Ca2+/Mg2+ were 1/0.6/0.07/0.2/0.26/0.4 for δβγ, 1/1.2/0.02/0.29/0.31/0.21 for αβγ, and 1/0.88/0.02/0.14/0.23/0.14 for δαβγ channels, respectively (75). In particular, the permeability to Li+ over Na+ ions for δαβγ ENaC was 0.88, which was distinguishable from both δβγ (1.2) and αβγ ENaC channels (0.6). Similar cation permeability was found for δ1βγ and δ2βγ channels (155).
Single-channel properties.
The single-channel Na+ ion conductance of δβγ ENaC was much higher than that of αβγ channels (12 vs. 4 pS), whereas there was no difference in their unitary Li+ conductance (8 pS) (72, 134). The unitary conductances of δαβγ channels were 8 pS for Na+ and 7.5 pS for Li+ ions (75). There were no subconductances observed. Moreover, the single-channel conductance was not altered by various ratios of cRNA injected between α and δ subunits. To date, three stoichiometric models have been proposed for ENaC channels: 2α1β1γ, 3α3β3γ, and 1α1β1γ (13, 39, 45, 70, 84, 115, 120, 121). These observations indicate that four ENaC subunits may stoichiometrically form new channels with a unique channel pore size. These data are supportive of the four-subunit and nine-subunit stoichiometric models. Considering the significant divergent sequence, tissue expression pattern, function, regulation, and species specificity between multiple subfamilies of the ENaC/DEG superfamily, the architecture of human δ ENaC channels may not be consistent with what has been found for the truncated chick ASIC1b (70). Even for ASIC channels, four subunits have been identified with variant expression patterns (78). Alternatively, δ ENaC may regulate the biophysical properties as an accessory protein but not as a pore-forming subunit. Another interpretation is that there may be complete electrical coupling between two channel populations, αβγ and δβγ channels, which appears as a single conductance. The unitary conductances of δ2βγ channels were 14.5 pS for Na+ and 9.8 pS for Li+ ions, which differ from the δ1βγ channels (155). A slight difference was seen by another group (138). The pre-second hydrophobic domain (H2) region in the δ subunit, actually part of the second transmembrane domain, contributes to both ion selectivity and single-channel conductance (73). This region is conserved between examined splice variants. We thus speculate that the diversity in the NH2-terminal tails may cause a conformational change and, in turn, affect single-channel conductance and ion selectivity possibly via altering channel subunit architecture, voltage dependence, cation affinity, or other unknown mechanisms (155).
The δ1βγ channels exhibit a long opening time compared with αβγ channels (68, 72). Furthermore, the estimated open probability value for δ2βγ was greater than that of δ1βγ (155). The larger unitary Na+ conductance and increased opening time apparently contribute to the greater macroscopic currents associated with δβγ channels. Wesch et al. (138), however, found a slight but not significant increment in the opening time of δ2 ENaC.
External Na+ self-inhibition.
An intrinsic feature of ENaC is that both native and cloned ENaC channels can be activated by either fast exposure from a solution containing low Na+ to Na+-rich bath solution or removal of channel blocker followed by an approximate half reduction in current level, namely, external Na+ self-inhibition (31, 35, 36, 52, 65, 89, 112). Human αβγ ENaC showed significant self-inhibition, which results in a lower open probability (short opening time) (59). δ ENaC-containing channels, including δ1βγ and δ1αβγ channels, did not exhibit significant self-inhibition compared with αβγ channels (59, 75). In strict contrast, the δ ENaC cloned from Xenopus laevis (ε xENaC) displayed strong self-inhibition, whereas αβγ xENaC did not (7).
Activation by extracellular protons.
Proton-activated currents in oocytes expressing δ subunit alone, δ+β, and δ+γ subunits were approximately −50 nA, which were amplified 44-fold by coexpressing δ with both β and γ subunits (71, 134, 148). An EC50 value of 6.0 for proton activation in δ and δβγ channels was observed. δ2βγ ENaC could enable proton activation as well as a faster response (145, 155). Protons may titrate pH-sensitive amino acid residues (His with a pKa of 6, Glu and Asp with a pKa of 4) residing in the extracellular loop, leading to a conformational change and disrupting the function of the degenerin sites (δS526, βS520, and γS529), eventually to extend the channel opening time (71).
δ ENaC channels have very slow activation and desensitization kinetics in response to a decrease in extracellular pH (71), in contrast with the fast activation and desensitization properties of most ASIC channels (5, 64, 153). The slow proton response would make δ ENaC channels good sensors of slow extracellular pH changes as may be found during ischemia.
Blockade by amiloride and analogs.
Amiloride, the first ENaC blocker to be applied to δβγ channels, has an IC50 of 2.6 μM (58, 71, 75, 134, 148). The Ki of amiloride for δβγ ENaC is 26-fold that of αβγ channels (0.1 μM for αβγ ENaC). Similarly, benzamil inhibited δβγ ENaC with an IC50 value 30-fold higher than that for αβγ channels (1, 134). Amiloride blockade of δβγ ENaC is much more voltage dependent compared with the αβγ channel (71). The Ki of amiloride for δαβγ channels was 920 and 13.7 μM at −120 and +80 mV, respectively, which significantly differed from that of both αβγ and δβγ channels (75). The splice variant δ2βγ channels were more sensitive to amiloride (Ki, 0.7 μM) and the blockade was not voltage dependent (155). In contrast, an earlier brief measurement did not detect significant difference in their IC50 values (14 μM for δ1βγ and 15 μM for δ2βγ) (147). Although the known amiloride binding site is located at the outer mouth of the channel, the NH2-terminal tail may indirectly regulate amiloride sensitivity through alterations in expression level, which influence the electrical field by changing Na+ gradient across plasma membrane. In addition, the current amplitude may affect the computation of amiloride sensitivity: the greater the whole-cell current, the more current fractions sensitive to the drug (with a smaller IC50 value) the channels. δ ENaC cloned from Xenopus laevis, so-called ε xENaC, had a 10-fold lower affinity to amiloride (7). δ ENaC was not sensitive to EIPA, a potent inhibitor of the Na+/H+ exchanger, at concentrations below 10 μM (134).
Inhibition by Evans blue.
Yamamura and coworkers (149) found that Evans blue was a specific antagonist of δβγ channels in Xenopus oocytes. Evans blue specifically inhibited human δβγ ENaC in a concentration-dependent manner (IC50, 143 μM). However, diverse observations were reported recently (155). Besides, this dye transiently activated human αβγ ENaC at doses less than 300 nM. Recently, Schwagerus and coworkers (111) even reported an incremental increase in transepithelial Na+ transport by Evans blue in δ ENaC-expressing human Calu-3 monolayer cells. These divergent observations may be caused by the experimental procedures applied by different groups. For example, preexposure of membrane-permeable amiloride could interact with Evans blue and modify the responses of ENaC proteins (149).
Activation by capsazepine.
Capsazepine, a competitive antagonist for transient receptor potential vanilloid subfamily 1 (TRPV1) (127), is the first reported activator of δ ENaC (144). Capsazepine elevated channel activity associated with δβγ ENaC 2.6-fold, with an EC50 of 7.8 μM (144). Weakly acidic pH (7.0) facilitated the activation of δβγ channels (EC50, 2.4 μM). In comparison, an increment to a less extent (1.6-fold) was observed in Xenopus oocytes expressing δ subunit alone. The authors postulated that β and γ subunits would amplify the stimulation by capsazepine. Recently these observations were confirmed in oocytes expressing δ1βγ and δ2βγ channels (155). The EC50 value of capsazepine for δ2βγ channels was approximately half that of δ1βγ channels. Clearly, capsazepine is a specific activator of δβγ channels. The inhibition of TRPV1 and αβγ ENaC channels, unfortunately, prevents the use of this compound as a potent pharmacological probe to investigate native δ ENaC channels. Additionally, the mechanisms for the activation of δβγ and inhibition of αβγ channels by capsazepine are still unknown.
Stimulation by S3969.
S3969, a small molecule, activated human δβγ ENaC in Xenopus oocytes with “comparable” EC50 value of 1.2 μM for δ1βγ and 0.4 μM for δ2βγ channels via increased opening time (91). It did not show the same stimulatory effects on mouse ENaC but stimulated human αβγ channels with identical potency, excluding the possibility that it acts as a specific agonist for δβγ channels.
Augmentation by icilin.
Icilin is a tetrahydropyrimidine-2-one derivative used as a cooling agent. Icilin was identified as an agonist to markedly enhance the activity of δ and δβγ heteromultimers expressed in Xenopus oocytes with an EC50 value of 33 μM (146). Icilin synergistically stimulated δβγ channels with protons or capsazepine (146). In contrast, the activity of αβγ ENaC channels was slightly inhibited. This stimulation was abolished by amiloride or absence of external sodium. These interesting observations have not been verified by other groups to date.
Cyclic nucleotides release self-inhibition.
Cell-permeable cpt-cAMP has long been used as an activator of the cAMP/PKA signaling pathway to evoke ENaC activity (22–24, 29, 32, 128). Surprisingly, this compound also serves as an external ligand to activate ENaC by releasing its self-inhibition. Molina et al. (98) reported that cpt-cAMP stimulated δβγ ENaC up to approximately threefold, and αβγ ENaC by twofold. Coexpression of δ ENaC with αβγ channels conferred cpt-cAMP-mediated activation with an EC50 value of 30 μM, similar to that for αβγ channels (49 μM).
We recently found that 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate-Na (CPT-cGMP) stimulated human αβγ ENaC in Xenopus oocytes in a PKG-independent and domain-specific manner (101). CPT-cGMP but not parent cGMP acutely activated human ENaC activity as an external ligand (99, 101). CPT-cGMP activated δαβγ ENaC with an EC50 of 5 and 225 μM, respectively, for inward (at −100 mV) and outward (at +40 mV) currents (99). Consistent with these observations, the EC50 of CPT-cGMP for activating transepithelial short-circuit currents in H441 monolayer cells was 147 μM. Collectively, the data indicate that both cpt-cAMP and CPT-cGMP can serve as an external ligand to activate ENaC channels in addition to stimulating the PKA/PKG signaling pathways.
Other compounds.
δ ENaC has also been found to be regulated by salt-taste-modifying reagents and acidic metabolites. Please see the following sections for details.
Physiological Regulation and Function
Fluid absorption.
Chimpanzee δ ENaC was first identified from kidney and deposited into NCBI by Dr. Eaton's group. Two δ ENaC transcripts were detected in the human alveolar epithelial cell line A549, primary human alveolar type cells, and human lung tissues (75, 99). δ2 ENaC was predominantly expressed in alveolar epithelial cells and coexpressed with δ1 in some cells. The physical intermolecular cross-talk between δ and the other three ENaC subunits indicates the potential existence of δ ENaC-containing channels in pulmonary epithelial cells (75). Indeed, δ ENaC channels contributed to ∼50% of amiloride-sensitive salt transport across primary human nasal epithelial cells (8). Considering the inconsistent observations of the effects of Evans blue on heterologous ENaC channels (155), additional evidence in basolateral membrane-permeabilized primary bronchoalveolar monolayer cells, and gene manipulation with siRNA specifically against δ ENaC will be needed to confirm these interesting observations. In addition, δ ENaC was also detected in human mesothelial cells, which play a key role in balancing the turnover and reabsorption of pleural fluid (74, 100). However, the contribution of δ ENaC to lung diseases is not known (6, 56, 92).
Apically located ENaC channels functionally integrate with K+ channels, Cl− channels, and Na+-K+-ATPase (46, 47, 51, 102, 107, 110, 141, 154). The native lung epithelial sodium channels are regulated by hormones, protein kinases, oxygen, cytokines/chemokines, etc. (17, 21, 30, 33, 34, 38, 50, 57, 62, 83, 93, 106, 116, 117, 126, 140, 151). Whether δ ENaC responds to these signals identical to those of α ENaC channels is still an open question.
Mechanosensor.
Recently, the critical role of ENaC in sensing tension in cardiovascular homeostasis was extensively reviewed (40, 41). Laminar shear stress modulates the activity of δβγ and αβγ channels by increasing channel opening time (2, 27), even though the response to flow of the δβγ ENaC channels was not as strong as that of αβγ channels (1). The flow sensors, most likely residing in the diverse second transmembrane region (M2) and the preceding region (H2), may lead to a conformational change in ENaC molecules and eventually increase the probability of the channel being open. Gating modulators, e.g., acidic pH and serine protease, eliminated the responses of both δ1βγ and δ2βγ heteromultimers (48). However, the contribution of δ ENaC to mechanotransduction in mammalian muscle spindles still remains inconclusive (114), since SCNN1D is a pseudogene in rat.
Salt taste receptors.
δ ENaC may be a component of the salt receptor (18). Using RT-PCR and in situ hybridization, Stahler and colleagues (119) found that the mRNA of δ ENaC was amplified in human circumvallate and fungiform tissues as well as in nonchemosensory lingual epithelium. Immunochemical localization showed that δ ENaC was expressed in all visible taste pores in fungiform papillae and keratinocytes of the epithelium surrounding the fungiform taste buds. To examine the function of ENaC subunits in salt taste perception, the authors examined the effects of salt-taste-modulating agents on human δβγ channels in oocytes. l-Arginine, l-lysine, l-homoarginine, and choline chloride activated δβγ channels in a concentration-dependent, reversible manner. Interestingly, l-arginine and homoarginine showed more potent effects on δβγ over αβγ channels.
Sour taste receptors.
δ ENaC was detected at the transcriptional and protein levels in human sour-normal and ageusic patients (68). δ ENaC was located in both apical and basolateral membranes in taste buds (68, 119). Given its presence in many taste cells of human fungiform papillae, and its activation by protons and other acidic compounds, δ ENaC is considered to be a candidate gene for sour taste reception as well as saltiness perception.
The splice variant δ2βγ may be a more effective pH sensor than δ1βγ on the basis of the increased gating kinetics and enhanced channel activity (155). In contrast, an insignificant increase in pH sensitivity in δ2βγ channels was reported in cells pretreated with amiloride (138). The preexposed amiloride might not be washed away completely, or amiloride binding-induced conformational change could not be restored in a few minutes. This membrane-permeable compound would exert noninhibitory effects intracellularly. Preapplication of amiloride to oocytes also caused inconsistent observations for the effects of Evans blue on both αβγ and δβγ channels (149, 155). In addition, δ ENaC may be a terminal acid receptor in human skin and gastrointestinal system and mediate the release of ATP (143, 145, 150). Carbon monoxide may be able to reduce δ ENaC activity (3).
Action potential.
δ ENaC alone or channels composed of this subunit displayed a constitutive channel activity. A persistent Na+ influx would depolarize the resting membrane potential and amplify the effect on synaptic potentials. It is conceivable that, working together with other ion channels, δ ENaC may trigger an action potential by gradually depolarizing membrane potential in neural cells. The distribution of δ ENaC in neurons in human brain supports its role in controlling membrane potential and cell excitability (139, 147). Acid-sensing ion channel 1a in the central nervous system has been implicated in long-term potentiation, suggesting that minute fluxes in synaptic pH may activate proton-sensitive δ ENaC channels to enhance postsynaptic plasticity, learning, and memory (15, 137, 144).
Sperm motility.
ENaC subunits are present in the flagellar midpiece and the acrosome of spermatogenic cells and mature sperm, respectively (63, 82). δ ENaC contributes to hyperpolarization of the sperm plasma membrane during capacitation, which is required to render sperm competent for fertilization (63). Blockade of ENaC channel activity significantly improved the movement of human sperm (82). δ ENaC apparently contributes to capacitation-associated hyperpolarization, in turn affecting the motility of human sperm. However, other mechanisms may be involved in mouse sperms based on the absence of δ ENaC in murine.
Heterogeneity of cation channels.
There are at least three subtypes of Na+-permeable cation channels in epithelial tissues (42, 52, 94). The highly Na+-selective channels have been attributed to channels made of α, β, and γ ENaC subunits. The molecular basis of the other two groups, however, remains in dispute. Although α ENaC channels and channels in nonpolarized cells exhibit features similar to the native moderate and less selective cation channels (69, 88, 152), these do not exist under physiological conditions. Native epithelial Na+ channels are assumed to be trimeric protein complexes in polarized salt-absorptive epithelial cells (70). Of note, homomultimeric channels composed of pore-forming α or δ ENaC subunit in Xenopus oocytes display similar biophysical and pharmacological properties, e.g., response to acidic pH (71, 78). The existence of δ ENaC and variants may partially explain the diverse phenotypes of amiloride-inhibitable channels in epithelial tissues. δ2 ENaC does not coexpress with its δ1 counterpart in all alveolar cells, indicating that δ2 is not a simple surrogate or alternative.
In glial cells, δ ENaC is required for syntaxin 1A to downregulate ASIC activity as shown by their physical interaction (14). δ ENaC reduced the activity of channels composed of ASIC1 and ASIC2 subunits in the presence of syntaxin 1A by reducing channel opening time (14). In addition, δ ENaC significantly extended the activation and inactivation kinetics (desensitization), reduced the differentiation between Na+ and K+ ions, and increased the cooperativity in PcTX1 venom binding sites when coexpressed with ASIC1 (96). δ and γ ENaC share similar and widespread distribution, with similar expression levels across various tissues. δ ENaC may be substituted for γ ENaC in normal brain tissue to form heteromultimeric Na+ channels with ASIC members, as supported by classic studies (76, 77). Of note, the current magnitude was not identical between heteromeric αβγ and αβδ channels (12). Furthermore, δ subunit may modify endocytosis and exocytosis of ENaC channel complexes (5a).
Proteolysis.
ENaC can be fully activated by serine proteases via proteolytic cleavage in α and γ subunits (see prior classic reviews for details; Refs. 80, 103, 109). Three potential cleavage sites have been proposed. Proteases cleave the extracellular loop of ENaC and release a short inhibitory peptide increasing channel open probability. Haerteis and colleagues (58) demonstrated that δ ENaC altered proteolysis of other subunits by serine proteases. The δ subunit itself may not be proteolytically cleaved by endogenous protease in the presence of β and γ subunits. Instead, δ ENaC promoted the cleavage of γ ENaC in the presence of β subunit to augment the channel activity of δβγ ENaC. In sharp contrast to the endogenous protease (trypsin), exogenous chymotrypsin cleaved the full-length heterologous δ ENaC (85 kDa) into two segments (65 and 20 kDa).
Regulation by Murr1.
Mouse U2af1-rs1 region (Murr1) is a 21-kDa protein mutated in Bedlington terriers suffering from copper toxicosis. Murr1 interacts physically with the COOH-terminal domain of δ ENaC (16). Murr1, when coexpressed with δβγ ENaC in Xenopus oocytes, augmented channel activity in a dose-dependent manner. It is proposed that Murr1 may regulate the transport of sodium and copper across epithelia as observed in fish gill and intestinal epithelium (60) and trafficking of both ENaC and copper transporters. Indeed, a follow-up study demonstrated that Murr1 increased the expression of δ ENaC proteins at the plasma membrane (28). This was substantiated by a recent finding that Murr1 specifically interacted with phosphatidylinositols, particularly Ptdlns(4,5)P2, a well-known signaling pathway for ENaC, and directed exocytosis of proteins in polarized cells (25). Murr1 may thereby serve as a scaffold to recruit δ ENaC to the luminal membrane of epithelial cells. δ ENaC ubiquitination is enhanced by Murr1, leading to augmented internalization (28). Please see the recent review on the trafficking and ubiquitination of ENaC (43).
Regulation by neuronal-specific serum- and glucocorticoid-induced kinase 1.1 (SGK1.1) and phospholipase C. Wesch et al. (139) recently reported that δ ENaC isoforms and neuronal SGK1.1 were coexpressed in pyramidal neurons of the human and monkey cerebral cortex. Coexpression of wild type SGK1.1 but not its enzymatic dead mutant in Xenopus oocytes increased amiloride-sensitive currents associated with δ1βγ and δ2βγ channels approximately threefold. The same amplifying magnitude was observed in channels comprised of δ1 or δ2 subunit alone, indicating that the SGK1.1 exerts its effect independent of the PY motifs. Furthermore, SGK1.1 action also depended on its binding to phosphatidylinositol(4,5)-bisphosphate. Deletion of PtdIns(4,5)P2 binding motif in SGK1.1 and activation of PLC by 3M3FBS through lysophosphatidic acid G protein-coupled receptors removed SGK1.1 from the plasma membrane and eliminated activation of δβγ channels. These data strongly support the upregulation of δ ENaC channels by the SGK1.1/PLC signal.
Divalent cations.
Hypotonic exposure of oocytes increased proton-activated currents by 92% in Xenopus oocytes expressing δβγ ENaC (71). A combination of hypotonicity and lactate synergistically stimulates proton-activated δβγ ENaC. Gating of ASIC channels by extracellular protons is regulated by intracellular and extracellular Ca2+ and Mg2+ ions (113, 135). Membrane-permeable Ca2+ chelator, BAPTA-AM (50 μM), in the absence of extracellular Ca2+ reduced the basal but not the proton-gated peak currents. EDTA, but not EGTA, can augment the response to hypotonicity and lactic acid ∼2.5-fold. In the presence of both EDTA and lactate, the increased proton-activated currents were stably elicited by repeated application of acidic solution, whereas the incremental proton-activation by either hypotonicity or lactate ran down in a few minutes. These results indicate that the extracellular Ca2+-to-Mg2+ ratio rather than Ca2+ alone affects the proton-induced currents of δβγ channels (71).
Pathological Relevance
Genetic diseases.
The SCNN1D gene is located on the plus strand of chromosome 1, from 1.21 to 1.23 Mb. Any terminal distal nonlethal deletion of 1p36 of a size larger than 1.3 Mb will lead to loss of this gene. The terminal distal part of chromosome 1 is lost in some rare diseases, e.g., monosomy 1p36 deletion syndrome (79). This delineated contiguous gene defect is characterized by distinct craniofacial features, associated with developmental delay/mental retardation, hypotonia, muscle hypotrophy, seizures, brain abnormalities, and heart diseases (10, 49). Roughly two-thirds of children with the 1p36 deletion suffer from recurrent respiratory infection (132). On the other hand, 1p36 in autism patients is truncated from 1.19 to 1.23 Mb (133). This region encodes both SCNN1D and UBE2J2 genes. Another atypical deletion of the Williams-Beuren syndrome chromosomal interval also leads to autism (44). Interestingly, 100% of autistic patients had doublets in the lower airway (122). Furthermore, gain of copies of SCNN1D genes have been noted in cardiovascular diseases (104), whereas 404 single nucleotide polymorphisms of SCNN1D have been collected in the NCBI database to date, although corresponding phenotypes have not yet been identified.
Ischemic disorders.
Regulation of the proton-activated currents of δβγ ENaC by hypoosmolarity and EDTA suggested that δ ENaC may integrate ischemia-related signals in inflamed and hypoxic tissues (71, 75). Proton sensitivity of δβγ ENaC may be an important mechanism for integrating external ischemic signals in inflamed and hypoxic tissues (71, 125). Lactic, pyruvic, and formic acids are generally produced during cardiac and brain ischemia and result in acidosis within minutes (85, 113). Facilitation of proton-activated currents by these acidic metabolites strongly indicates that δ ENaC is most likely involved in ischemic signal transduction. ASIC channels are thought to be activated to transduce ischemia-related signals. However, pH decrement generally requires minutes to develop. The millisecond activation of ASIC may be an additional limitation for ASIC as effective pH sensors. Compared with ASIC channels, δ ENaC is distinguished by a very slow activation and desensitization process, allowing ischemic cells to have enough time to detect changes in proton concentration and osmolarity. Another striking feature of δ ENaC is its slow desensitization: the ASIC channel generally desensitized in less than a second (135), whereas δ ENaC took minutes (71). Thus the gating properties of δ ENaC indicated that, as an ischemic sensor, it differs from ASIC channels. Pulmonary δβγ ENaC may also serve as a pH sensor in response to accidental noxious acidic aspiration and post occupational exposure.
Neurological diseases.
Alternative gene transcript splicing produces multiple proteins as mentioned previously. The INTRON2 of SCNN1D had a 52% more variants in human brain of mesial temporal lobe epilepsy (61). δ ENaC proteins in human nonneoplastic brain tissues were more abundant than in glioblastoma patients (77). Therefore, the absence of δ ENaC may be related to alterations in the channel complex of glioblastoma patients (76, 77). The mRNA of δ ENaC has not been found in glial cells in the normal human brain (53).
Abnormal expression and associated phenotypes.
Using combined laser-capture microdissection and RNA array, Gronich et al. (55) reported a 1.6-fold reduction of SCNN1D in ventricular myocytes of ischemic cardiomyopathy sufferers/patients. Decreased expression of δ ENaC may contribute to disrupted Na+ and K+ homeostasis in ischemic heart diseases. Global gene expression analysis found that chemotherapy reduced the expression of δ ENaC in human ovarian cancer spheroids (87). According to NCBI GEO profiles, a significant reduction in the expression of δ ENaC was also found in the following diseases: diabetic visceral adipose tissue, atypical ductal hyperplasmia combined with breast cancer, hereditary gingival fibromatosis, neural tube defects, tumorigenic breast cancer, skeletal muscle following weight loss, COPD macrophages, utero preeclampsia, Duchenne muscular dystrophy, carboplatin-sensitive ovarian carcinoma, and acute malarial infection. In vitro, the expression of δ ENaC was depressed under these culture conditions: hypoxic B lymphocyte, ultrafine particle-treated endothelial cells, time-dependent reduction in serum treated fibroblasts, INF-γ-treated keratinocytes, homocysteine-exposed aortic smooth muscle cells, TGF-β1-treated HK2 proximal tubular cells, and immortalized endothelial cells.
On the other hand, an incremental increase in the expression of δ ENaC under the following conditions has been shown in the NCBI GEO profiles: cells overexpressing HCaRG, K562 leukemic cells (CD34+) exposed to imatinib, small airway epithelial cells of cigarette smokers, CREB-depleted myeloid leukemic cells, diabetic nephropathy, heart of low-Na+ dieter, optineurin-depleted HeLa cells, sperm cells of teratozoospermia, and fibroblast cells in desmoid tumors (19).
Potential biomarker of kidney diseases.
Human urinary microvesicles collected solely from the medullary thick ascending limb of Henle contain δ ENaC mRNA (97). SCNN1D has therefore been proposed as a potential novel and noninvasive biomarker of renal diseases.
Prospective Considerations
In the aggregate, the current literature suggests that δ ENaC plays an important role in normal and diseased tissues. Given the lack of this gene in mice, its precise physiological function and phenotype in deficient animals cannot be evaluated in a mouse model. α ENaC deficiency can cause death of mice at birth resulted from air space flooding most likely due to lack of compensation by δ ENaC. The most promising findings are from human primary nasal epithelial cells, showing that up to 40% of amiloride-sensitive sodium transport is associated with δ ENaC (8). On the other hand, children with genetic deletion of SCNN1D genes are predisposed to respiratory infection and the development of nasal congestion during the winter months (132). In contrast, the major phenotype associated with deletion of SCNN1A in human 12p13 is growth retardation resulted from urinary salt wasting (9, 131). Whether δ ENaC is an essential pathway of Na+ reabsorption in lung and kidney awaits further in vivo and ex vivo studies in primates. Developing gene knockout animal models will help clarify the physiological role of δ ENaC in nonepithelial tissues, including the brain and heart. (Table 3)
Table 3.
Top 10 human cell types and cell lines expressing SCNN1D transcripts, and top 10 human diseases with significant alteration in RNA expression level (NextBio.com)
| Tissue types (Median 315) | Peripheral blood reticulocytes (1160) > Cord blood reticulocytes (1080) > Peripheral blood B lymphocytes (naive) (1000) > Peripheral blood B lymphocytes (memory) (869) > Epidermal transit amplifying cells (841) > Epidermal stem cells (774) > Synaptoneurosomes from the frontal cortex (752) > Peripheral blood leukocytes (732) > Plasma cells/centrocytes from tonsils (720) > Granulocyte-macrophage progenitor cells from bone marrow (676) |
| Cell lines (Median 243) | Large cell lung carcinoma cell line HCC 1359 (642) > Lung adenocarcinoma cell line HCC2006 (513) > Small cell lung carcinoma cell line NCI-H889 (493) > Bladder carcinoma cell line UM-UC-9 (457) > Mesothelioma cell line NCI-H28 (443) > Liver carcinoma cell line C3A (430) > Ovarian adenocarcinoma cell line OVCAR-5 (424) > Small cell lung carcinoma cell line DMS 114 (423) > Squamous cell lung carcinoma cell line HCC1171 (422) > Lung carcinoma cell line NCI-H227 (418) |
| Diseases associated with increased expression (0–100%) | Spinocerebellar ataxia (100) > Mental disorder (99) > Encephalomyelopathy/Idiopathic thrombocytopenic purpura (82) > Developmental mental disorder (72) > Anxiety disorder (66) > Delayed hypersensitivity disorder (55) > Platelet count below reference range (54) > Rheumatoid arthritis (52) > Chronic sinusitis (50) > Allergic disorder (47) |
| Diseases associated with reduced expression (0–100%) | Cardiac transplant rejection/ Gastrointestinal hemorrhage/Neuroendocrine tumor (70) > Cholestasis (65) > Brain cancer (64) > Malignant head and neck tumor (63) > Epstein-Barr virus infection (61) > Malignant tumor of intestine (57) > Rhinovirus diseases (55) > Viral infections of the central nervous system (52) > Osteoporosis/Meningitis/Gastritis (51) > Picornavirus diseases (50) |
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL87017, HL031197, and HL095435.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.-L.J. conception and design of research; H.-L.J., R.-Z.Z., and Z.-X.C. prepared figures; H.-L.J., R.-Z.Z., S.S., S.I., and S.M. drafted manuscript; H.-L.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Nie Hong-Guang for suggestive discussion.
REFERENCES
- 1. Abi-Antoun T, Shi S, Tolino LA, Kleyman TR, Carattino MD. Second transmembrane domain modulates epithelial sodium channel gating in response to shear stress. Am J Physiol Renal Physiol 300: F1089–F1095, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J 21: 2389–2399, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Althaus MFM, Buchaekert Y, Vadasz I, Clauss WG, Seeger W, Motterlini R, Morty RE. Carbon monooxide modulates the activity of sodium channels in the alveolar epithelium: a role for δENaC? (Abstract). Am J Respir Crit Care Med 179: A4950, 2009. [Google Scholar]
- 4. Alvarez de la Rosa D, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Annu Rev Physiol 62: 573–594, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA 99: 2326–2331, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a. Alvarez de la Rosa D, Wesch DL, Miranda P, Giraldez T. Epithelial sodium channel (ENaC) plasma membrane turnover is modified in channels containing δ subunits (Abstract). FASEB J 26: 1068, 2012. [Google Scholar]
- 6. Azzam ZS, Adir Y, Welch L, Chen J, Winaver J, Factor P, Krivoy N, Hoffman A, Sznajder JI, Abassi Z. Alveolar fluid reabsorption is increased in rats with compensated heart failure. Am J Physiol Lung Cell Mol Physiol 291: L1094–L1100, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Babini E, Geisler HS, Siba M, Grunder S. A new subunit of the epithelial Na+ channel identifies regions involved in Na+ self-inhibition. J Biol Chem 278: 28418–28426, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Bangel-Ruland N, Sobczak K, Christmann T, Kentrup D, Langhorst H, Kusche-Vihrog K, Weber WM. Characterization of the epithelial sodium channel δ-subunit in human nasal epithelium. Am J Respir Cell Mol Biol 42: 498–505, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Baroncini A, Avellini C, Neri C, Forabosco A. Distal 12p deletion in a stillborn infant. Am J Med Genet 36: 358–360, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Battaglia A. Del 1p36 syndrome: a newly emerging clinical entity. Brain Dev 27: 358–361, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol 520: 631–644, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berdiev BK, Jovov B, Tucker WC, Naren AP, Fuller CM, Chapman ER, Benos DJ. ENaC subunit-subunit interactions and inhibition by syntaxin 1A. Am J Physiol Renal Physiol 286: F1100–F1106, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Berdiev BK, Karlson KH, Jovov B, Ripoll PJ, Morris R, Loffing-Cueni D, Halpin P, Stanton BA, Kleyman TR, Ismailov II. Subunit stoichiometry of a core conduction element in a cloned epithelial amiloride-sensitive Na+ channel. Biophys J 75: 2292–2301, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem 278: 15023–15034, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Bianchi L, Driscoll M. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron 34: 337–340, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Biasio W, Chang T, McIntosh CJ, McDonald FJ. Identification of Murr1 as a regulator of the human δ epithelial sodium channel. J Biol Chem 279: 5429–5434, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Boncoeur E, Tardif V, Tessier MC, Morneau F, Lavoie J, Gendreau-Berthiaume E, Grygorczyk R, Dagenais A, Berthiaume Y. Modulation of epithelial sodium channel activity by lipopolysaccharide in alveolar type II cells: involvement of purinergic signaling. Am J Physiol Lung Cell Mol Physiol 298: L417–L426, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Boughter JD, Jr, Gilbertson TA. From channels to behavior: an integrative model of NaCl taste. Neuron 22: 213–215, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Bowden NA, Croft A, Scott RJ. Gene expression profiling in familial adenomatous polyposis adenomas and desmoid disease. Hered Cancer Clin Pract 5: 79–96, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol Cell Physiol 283: C126–C134, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Brown SG, Gallacher M, Olver RE, Wilson SM. The regulation of selective and nonselective Na+ conductances in H441 human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L942–L954, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Bubien JK, Cornwell T, Bradford AL, Fuller CM, DuVall MD, Benos DJ. α-Adrenergic receptors regulate human lymphocyte amiloride-sensitive sodium channels. Am J Physiol Cell Physiol 275: C702–C710, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Bubien JK, Ismailov II, Berdiev BK, Cornwell T, Lifton RP, Fuller CM, Achard JM, Benos DJ, Warnock DG. Liddle's disease: abnormal regulation of amiloride-sensitive Na+ channels by β-subunit mutation. Am J Physiol Cell Physiol 270: C208–C213, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Bubien JK, Watson B, Khan MA, Langloh AL, Fuller CM, Berdiev B, Tousson A, Benos DJ. Expression and regulation of normal and polymorphic epithelial sodium channel by human lymphocytes. J Biol Chem 276: 8557–8566, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Burkhead JL, Morgan CT, Shinde U, Haddock G, Lutsenko S. COMMD1 forms oligomeric complexes targeted to the endocytic membranes via specific interactions with phosphatidylinositol 4,5-bisphosphate. J Biol Chem 284: 696–707, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Chang T, Ke Y, Ly K, McDonald FJ. COMMD1 regulates the δ epithelial sodium channel (δENaC) through trafficking and ubiquitination. Biochem Biophys Res Commun 411: 506–511, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Chen L, Song W, Davis IC, Shrestha K, Schwiebert E, Sullender WM, Matalon S. Inhibition of Na+ transport in lung epithelial cells by respiratory syncytial virus infection. Am J Respir Cell Mol Biol 40: 588–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen XJ, Eaton DC, Jain L. β-Adrenergic regulation of amiloride-sensitive lung sodium channels. Am J Physiol Lung Cell Mol Physiol 282: L609–L620, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Chraibi A, Horisberger JD. Na self-inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol 120: 133–145, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chraibi A, Schnizler M, Clauss W, Horisberger JD. Effects of 8-cpt-cAMP on the epithelial sodium channel expressed in Xenopus oocytes. J Membr Biol 183: 15–23, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Collett A, Ramminger SJ, Olver RE, Wilson SM. β-Adrenoceptor-mediated control of apical membrane conductive properties in fetal distal lung epithelia. Am J Physiol Lung Cell Mol Physiol 282: L621–L630, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Correa-Meyer E, Pesce L, Guerrero C, Sznajder JI. Cyclic stretch activates ERK1/2 via G proteins and EGFR in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 282: L883–L891, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Cucu D, Simaels J, Eggermont J, Van Driessche W, Zeiske W. Opposite effects of Ni2+ on Xenopus and rat ENaCs expressed in Xenopus oocytes. Am J Physiol Cell Physiol 289: C946–C958, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Cucu D, Simaels J, Van Driessche W, Zeiske W. External Ni2+ and ENaC in A6 cells: Na+ current stimulation by competition at a binding site for amiloride and Na+. J Membr Biol 194: 33–45, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Dijkink L, Hartog A, van Os CH, Bindels RJ. The epithelial sodium channel (ENaC) is intracellularly located as a tetramer. Pflügers Arch 444: 549–555, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Dagenais A, Frechette R, Clermont ME, Masse C, Prive A, Brochiero E, Berthiaume Y. Dexamethasone inhibits the action of TNF on ENaC expression and activity. Am J Physiol Lung Cell Mol Physiol 291: L1220–L1231, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology 23: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension 51: 1265–1271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 71: 403–423, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Eaton DC, Malik B, Bao HF, Yu L, Jain L. Regulation of epithelial sodium channel trafficking by ubiquitination. Proc Am Thorac Soc 7: 54–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edelmann L, Prosnitz A, Pardo S, Bhatt J, Cohen N, Lauriat T, Ouchanov L, Gonzalez PJ, Manghi ER, Bondy P, Esquivel M, Monge S, Delgado MF, Splendore A, Francke U, Burton BK, McInnes LA. An atypical deletion of the Williams-Beuren syndrome interval implicates genes associated with defective visuospatial processing and autism. J Med Genet 44: 136–143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC). EMBO J 17: 344–352, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fischer H, Illek B, Finkbeiner WE, Widdicombe JH. Basolateral Cl channels in primary airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol 292: L1432–L1443, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Fisher JL, Margulies SS. Na+-K+-ATPase activity in alveolar epithelial cells increases with cyclic stretch. Am J Physiol Lung Cell Mol Physiol 283: L737–L746, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Fronius M, Althaus M, Assmann M, Bednarz M, Clauss W. The δ1 and δ2 ENaC subunits form mechanosensitive channels when coexpressed with β and γ subunits (Abstract). Physiologist 54: A22 (8.3), 2011. [Google Scholar]
- 49. Gajecka M, Mackay KL, Shaffer LG. Monosomy 1p36 deletion syndrome. Am J Med Genet C Semin Med Genet 145C: 346–356, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Galietta LJ, Folli C, Marchetti C, Romano L, Carpani D, Conese M, Zegarra-Moran O. Modification of transepithelial ion transport in human cultured bronchial epithelial cells by interferon-γ. Am J Physiol Lung Cell Mol Physiol 278: L1186–L1194, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Gao L, Yankaskas JR, Fuller CM, Sorscher EJ, Matalon S, Forman HJ, Venglarik CJ. Chlorzoxazone or 1-EBIO increases Na+ absorption across cystic fibrosis airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1123–L1129, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Garty H, Benos DJ. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev 68: 309–373, 1988 [DOI] [PubMed] [Google Scholar]
- 53. Giraldez T, Afonso-Oramas D, Cruz-Muros I, Garcia-Marin V, Pagel P, Gonzalez-Hernandez T, Alvarez de la Rosa D. Cloning and functional expression of a new epithelial sodium channel δ subunit isoform differentially expressed in neurons of the human and monkey telencephalon. J Neurochem 102: 1304–1315, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Giraldez T, Rojas P, Jou J, Flores C, Alvarez de la Rosa D. The epithelial sodium channel δ-subunit: new notes for an old song. Am J Physiol Renal Physiol 303: F328–F338, 2012 [DOI] [PubMed] [Google Scholar]
- 55. Gronich N, Kumar A, Zhang Y, Efimov IR, Soldatov NM. Molecular remodeling of ion channels, exchangers and pumps in atrial and ventricular myocytes in ischemic cardiomyopathy. Channels (Austin) 4: 101–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guidot DM, Folkesson HG, Jain L, Sznajder JI, Pittet JF, Matthay MA. Integrating acute lung injury and regulation of alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol 291: L301–L306, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Guney S, Schuler A, Ott A, Hoschele S, Zugel S, Baloglu E, Bartsch P, Mairbaurl H. Dexamethasone prevents transport inhibition by hypoxia in rat lung and alveolar epithelial cells by stimulating activity and expression of Na+-K+-ATPase and epithelial Na+ channels. Am J Physiol Lung Cell Mol Physiol 293: L1332–L1338, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Haerteis S, Krueger B, Korbmacher C, Rauh R. The δ-subunit of the epithelial sodium channel (ENaC) enhances channel activity and alters proteolytic ENaC activation. J Biol Chem 284: 29024–29040, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Han DY, Nie HG, Su XF, Shi XM, Bhattarai D, Zhao M, Zhao RZ, Landers K, Tang H, Zhang L, Ji HL. 8-pCPT-cGMP stimulates human alveolar fluid clearance by releasing external Na+ self-inhibition of epithelial Na+ channels. Am J Respir Cell Mol Biol 45: 1007–1014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Handy RD, Eddy FB, Baines H. Sodium-dependent copper uptake across epithelia: a review of rationale with experimental evidence from gill and intestine. Biochim Biophys Acta 1566: 104–115, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Heinzen EL, Yoon W, Weale ME, Sen A, Wood NW, Burke JR, Welsh-Bohmer KA, Hulette CM, Sisodiya SM, Goldstein DB. Alternative ion channel splicing in mesial temporal lobe epilepsy and Alzheimer's disease. Genome Biol 8: R32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Helms MN, Chen XJ, Ramosevac S, Eaton DC, Jain L. Dopamine regulation of amiloride-sensitive sodium channels in lung cells. Am J Physiol Lung Cell Mol Physiol 290: L710–L722, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Hernandez-Gonzalez EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, Lopez-Gonzalez I, Demarco I, Wertheimer E, Darszon A, Visconti PE. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem 281: 5623–5633, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Hesselager M, Timmermann DB, Ahring PK. pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem 279: 11006–11015, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Horisberger JD, Chraibi A. Epithelial sodium channel: a ligand-gated channel? Nephron Physiol 96: 37–41, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Hummler E, Planes C. Importance of ENaC-mediated sodium transport in alveolar fluid clearance using genetically-engineered mice. Cell Physiol Biochem 25: 63–70, 2010 [DOI] [PubMed] [Google Scholar]
- 67. Hummler E, Rossier BC. Physiological and pathophysiological role of the epithelial sodium channel in the control of blood pressure. Kidney Blood Press Res 19: 160–165, 1996 [DOI] [PubMed] [Google Scholar]
- 68. Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman AI, Feldman RS, Mackler SA, Brand JG. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS ONE 4: e7347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol 280: L646–L658, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Ji HL, Benos DJ. Degenerin sites mediate proton activation of δβγ-epithelial sodium channel. J Biol Chem 279: 26939–26947, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Ji HL, Bishop LR, Anderson SJ, Fuller CM, Benos DJ. The role of Pre-H2 domains of α- and δ-epithelial Na+ channels in ion permeation, conductance, and amiloride sensitivity. J Biol Chem 279: 8428–8440, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Ji HL, Chalfant ML, Jovov B, Lockhart JP, Parker SB, Fuller CM, Stanton BA, Benos DJ. The cytosolic termini of the β- and γ-ENaC subunits are involved in the functional interactions between cystic fibrosis transmembrane conductance regulator and epithelial sodium channel. J Biol Chem 275: 27947–27956, 2000 [DOI] [PubMed] [Google Scholar]
- 74. Ji HL, Nie HG. Electrolyte and fluid transport in mesothelial cells. J Epithel Biol Pharmacol 1: 1–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ji HL, Su XF, Kedar S, Li J, Barbry P, Smith PR, Matalon S, Benos DJ. δ-subunit confers novel biophysical features to αβγ-human epithelial sodium channel (ENaC) via a physical interaction. J Biol Chem 281: 8233–8241, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM, Benos DJ. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem 284: 24526–24541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kapoor N, Lee W, Clark E, Bartoszewski R, McNicholas CM, Latham CB, Bebok Z, Parpura V, Fuller CM, Palmer CA, Benos DJ. Interaction of ASIC1 and ENaC subunits in human glioma cells and rat astrocytes. Am J Physiol Cell Physiol 300: C1246–C1259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Keppler-Noreuil KM, Carroll AJ, Finley WH, Rutledge SL. Chromosome 1p terminal deletion: report of new findings and confirmation of two characteristic phenotypes. J Med Genet 32: 619–622, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kleyman TR, Sheng S, Kosari F, Kieber-Emmons T. Mechanism of action of amiloride: a molecular prospective. Semin Nephrol 19: 524–532, 1999 [PubMed] [Google Scholar]
- 82. Kong XB, Ma HG, Li HG, Xiong CL. Blockade of epithelial sodium channels improve sperm motility in asthenospermia patients. Int J Androl 32: 330–336, 2009 [DOI] [PubMed] [Google Scholar]
- 83. Kooijman EE, Kuzenko SR, Gong D, Best MD, Folkesson HG. Phosphatidylinositol 4,5-bisphosphate stimulates alveolar epithelial fluid clearance in male and female adult rats. Am J Physiol Lung Cell Mol Physiol 301: L804–L811, 2011 [DOI] [PubMed] [Google Scholar]
- 84. Kosari F, Sheng S, Li J, Mak DO, Foskett JK, Kleyman TR. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem 273: 13469–13474, 1998 [DOI] [PubMed] [Google Scholar]
- 85. Kraig RP, Ferreira-Filho CR, Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol 49: 831–850, 1983 [DOI] [PubMed] [Google Scholar]
- 86. Krueger B, Schlotzer-Schrehardt U, Haerteis S, Zenkel M, Chankiewitz VE, Amann KU, Kruse FE, Korbmacher C. Four subunits (αβγδ) of the epithelial sodium channel (ENaC) are expressed in the human eye in various locations. Invest Ophthalmol Vis Sci 53: 596–604, 2012 [DOI] [PubMed] [Google Scholar]
- 87. L'Esperance S, Bachvarova M, Tetu B, Mes-Masson AM, Bachvarov D. Global gene expression analysis of early response to chemotherapy treatment in ovarian cancer spheroids. BMC Genomics 9: 99, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lazrak A, Samanta A, Venetsanou K, Barbry P, Matalon S. Modification of biophysical properties of lung epithelial Na+ channels by dexamethasone. Am J Physiol Cell Physiol 279: C762–C770, 2000 [DOI] [PubMed] [Google Scholar]
- 89. Li JH, Kau ST. Bumetanide stimulation of sodium permeability of the apical membrane of toad urinary bladder. J Pharmacol Exp Ther 246: 980–985, 1988 [PubMed] [Google Scholar]
- 90. Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett 318: 95–99, 1993 [DOI] [PubMed] [Google Scholar]
- 91. Lu M, Echeverri F, Kalabat D, Laita B, Dahan DS, Smith RD, Xu H, Staszewski L, Yamamoto J, Ling J, Hwang N, Kimmich R, Li P, Patron E, Keung W, Patron A, Moyer BD. Small molecule activator of the human epithelial sodium channel. J Biol Chem 283: 11981–11994, 2008 [DOI] [PubMed] [Google Scholar]
- 92. Mac Sweeney R, Fischer H, McAuley DF. Nasal potential difference to detect Na+ channel dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol 300: L305–L318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mairbaurl H, Mayer K, Kim KJ, Borok Z, Bartsch P, Crandall ED. Hypoxia decreases active Na transport across primary rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 282: L659–L665, 2002 [DOI] [PubMed] [Google Scholar]
- 94. Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: Biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol 93: 1852–1859, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Matthay MA, Clerici C, Saumon G. Invited review: Active fluid clearance from the distal air spaces of the lung. J Appl Physiol 93: 1533–1541, 2002 [DOI] [PubMed] [Google Scholar]
- 96. Meltzer RH, Kapoor N, Qadri YJ, Anderson SJ, Fuller CM, Benos DJ. Heteromeric assembly of acid-sensitive ion channel and epithelial sodium channel subunits. J Biol Chem 282: 25548–25559, 2007 [DOI] [PubMed] [Google Scholar]
- 97. Miranda KC, Bond DT, McKee M, Skog J, Paunescu TG, Da Silva N, Brown D, Russo LM. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int 78: 191–199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Molina R, Han DY, Su XF, Zhao RZ, Zhao M, Sharp GM, Chang Y, Ji HL. Cpt-cAMP activates human epithelial sodium channels via relieving self-inhibition. Biochim Biophys Acta 1808: 1818–1826, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nie HG, Chen L, Han DY, Li J, Song WF, Wei SP, Fang XH, Gu X, Matalon S, Ji HL. Regulation of epithelial sodium channels by cGMP/PKGII. J Physiol 587: 2663–2676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nie HG, Tucker T, Su XF, Na T, Peng JB, Smith PR, Idell S, Ji HL. Expression and regulation of epithelial Na+ channels by nucleotides in pleural mesothelial cells. Am J Respir Cell Mol Biol 40: 543–554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nie HG, Zhang W, Han DY, Li QN, Li J, Zhao RZ, Su XF, Peng JB, Ji HL. 8-pCPT-cGMP stimulates αβγ-ENaC activity in oocytes as an external ligand requiring specific nucleotide moieties. Am J Physiol Renal Physiol 298: F323–F334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. O'Grady SM, Jiang X, Ingbar DH. Cl− channel activation is necessary for stimulation of Na transport in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L239–L244, 2000 [DOI] [PubMed] [Google Scholar]
- 103. Planes C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol 78: 23–46, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Prakash SK, LeMaire SA, Guo DC, Russell L, Regalado ES, Golabbakhsh H, Johnson RJ, Safi HJ, Estrera AL, Coselli JS, Bray MS, Leal SM, Milewicz DM, Belmont JW. Rare copy number variants disrupt genes regulating vascular smooth muscle cell adhesion and contractility in sporadic thoracic aortic aneurysms and dissections. Am J Hum Genet 87: 743–756, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qadri YJ, Rooj AK, Fuller CM. ENaCs and ASICs as therapeutic targets. Am J Physiol Cell Physiol 302: C943–C965, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rafii B, Coutinho C, Otulakowski G, O'Brodovich H. Oxygen induction of epithelial Na+ transport requires heme proteins. Am J Physiol Lung Cell Mol Physiol 278: L399–L406, 2000 [DOI] [PubMed] [Google Scholar]
- 107. Rahman MS, Gandhi S, Otulakowski G, Duan W, Sarangapani A, O'Brodovich H. Long-term terbutaline exposure stimulates α1-Na+-K+-ATPase expression at posttranscriptional level in rat fetal distal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 298: L96–L104, 2010 [DOI] [PubMed] [Google Scholar]
- 108. Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002 [DOI] [PubMed] [Google Scholar]
- 109. Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol 71: 361–379, 2009 [DOI] [PubMed] [Google Scholar]
- 110. Rubenstein RC, Lockwood SR, Lide E, Bauer R, Suaud L, Grumbach Y. Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L88–L101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schwagerus E, Buckley S, Ehrhardt C. Expression and function of the epithelial sodium channel δ subunit in human respiratory epithelial cell lines (Abstract). Am J Respir Crit Care Med 183: A4229, 2011. [Google Scholar]
- 112. Sheng S, Perry CJ, Kleyman TR. Extracellular Zn2+ activates epithelial Na+ channels by eliminating Na+ self-inhibition. J Biol Chem 279: 31687–31696, 2004 [DOI] [PubMed] [Google Scholar]
- 113. Silver IA, Erecinska M. Ion homeostasis in rat brain in vivo: intra- and extracellular Ca2+ and H+ in the hippocampus during recovery from short-term, transient ischemia. J Cereb Blood Flow Metab 12: 759–772, 1992 [DOI] [PubMed] [Google Scholar]
- 114. Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol 588: 171–185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Snyder PM, Cheng C, Prince LS, Rogers JC, Welsh MJ. Electrophysiological and biochemical evidence that DEG/ENaC cation channels are composed of nine subunits. J Biol Chem 273: 681–684, 1998 [DOI] [PubMed] [Google Scholar]
- 116. Song Y, Namkung W, Nielson DW, Lee JW, Finkbeiner WE, Verkman AS. Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: dependence on Na+ and Cl− channel function. Am J Physiol Lung Cell Mol Physiol 297: L1131–L1140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Soukup B, Benjamin A, Orogo-Wenn M, Walters D. Physiological effect of protein kinase C on ENaC-mediated lung liquid regulation in the adult rat lung. Am J Physiol Lung Cell Mol Physiol 302: L133–L139, 2012 [DOI] [PubMed] [Google Scholar]
- 118. Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem 285: 30363–30369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stahler FRK, Demgensky S, Neumann K, Dunkel A, Taubert A, Raab B, Behrens M, Raguse J, Hofmann T, Meyerhof W. A role of the epithelial sodium channel in human salt taste transduction? Chem Percept 1: 78–90, 2008 [Google Scholar]
- 120. Staruschenko A, Adams E, Booth RE, Stockand JD. Epithelial Na+ channel subunit stoichiometry. Biophys J 88: 3966–3975, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Staruschenko A, Medina JL, Patel P, Shapiro MS, Booth RE, Stockand JD. Fluorescence resonance energy transfer analysis of subunit stoichiometry of the epithelial Na+ channel. J Biol Chem 279: 27729–27734, 2004 [DOI] [PubMed] [Google Scholar]
- 122. Stewart B. Can bronchoscopic airway anatomy be an indicator of autism? (Abstract). Chest 140: 388A, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life 60: 620–628, 2008 [DOI] [PubMed] [Google Scholar]
- 124. Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 101: 6062–6067, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Su X, Li Q, Shrestha K, Cormet-Boyaka E, Chen L, Smith PR, Sorscher EJ, Benos DJ, Matalon S, Ji HL. Interregulation of proton-gated Na+ channel 3 and cystic fibrosis transmembrane conductance regulator. J Biol Chem 281: 36960–36968, 2006 [DOI] [PubMed] [Google Scholar]
- 126. Su X, Robriquet L, Folkesson HG, Matthay MA. Protective effect of endogenous β-adrenergic tone on lung fluid balance in acute bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 290: L769–L776, 2006 [DOI] [PubMed] [Google Scholar]
- 127. Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51: 159–212, 1999 [PubMed] [Google Scholar]
- 128. Tamba K, Oh YS, Tucker JK, Quick MW, Warnock DG. Epithelial sodium channels expressed in Xenopus oocytes are activated by cyclic-AMP. Clin Exp Nephrol 6: 195–201, 2002 [Google Scholar]
- 129. Teiwes J, Toto RD. Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens 20: 109–117, 2007 [DOI] [PubMed] [Google Scholar]
- 130. Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol 7, Suppl 1: S12.1–14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Trautmann U, Pfeiffer RA. Interstitial deletion 12p13.1–13.3 in a mildly retarded infant with unilateral ectrodactyly. Ann Genet 37: 147–149, 1994 [PubMed] [Google Scholar]
- 132. Unique 1p36 deletion syndrome. Available from: http://www.rarechromo.org/information/Chromosome%20%201/1p36%20deletion%20FTNW.pdf
- 133. van der Zwaag B, Franke L, Poot M, Hochstenbach R, Spierenburg HA, Vorstman JA, van Daalen E, de Jonge MV, Verbeek NE, Brilstra EH, van 't Slot R, Ophoff RA, van Es MA, Blauw HM, Veldink JH, Buizer-Voskamp JE, Beemer FA, van den Berg LH, Wijmenga C, van Amstel HK, van Engeland H, Burbach JP, Staal WG. Gene-network analysis identifies susceptibility genes related to glycobiology in autism. PLoS ONE 4: e5324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem 270: 27411–27414, 1995 [DOI] [PubMed] [Google Scholar]
- 135. Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann NY Acad Sci 868: 67–76, 1999 [DOI] [PubMed] [Google Scholar]
- 136. Waldmann R, Voilley N, Mattei MG, Lazdunski M. The human degenerin MDEG, an amiloride-sensitive neuronal cation channel, is localized on chromosome 17q11.2–17q12 close to the microsatellite D17S798. Genomics 37: 269–270, 1996 [DOI] [PubMed] [Google Scholar]
- 137. Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34: 463–477, 2002 [DOI] [PubMed] [Google Scholar]
- 138. Wesch D, Althaus M, Miranda P, Cruz-Muros I, Fronius M, Gonzalez-Hernandez T, Clauss WG, Alvarez de la Rosa D, Giraldez T. Differential N termini in epithelial Na+ channel δ-subunit isoforms modulate channel trafficking to the membrane. Am J Physiol Cell Physiol 302: C868–C879, 2012 [DOI] [PubMed] [Google Scholar]
- 139. Wesch D, Miranda P, Afonso-Oramas D, Althaus M, Castro-Hernandez J, Dominguez J, Morty RE, Clauss W, Gonzalez-Hernandez T, Alvarez de la Rosa D, Giraldez T. The neuronal-specific SGK1.1 kinase regulates δ-epithelial Na+ channel independently of PY motifs and couples it to phospholipase C signaling. Am J Physiol Cell Physiol 299: C779–C790, 2010 [DOI] [PubMed] [Google Scholar]
- 140. Willis BC, Kim KJ, Li X, Liebler J, Crandall ED, Borok Z. Modulation of ion conductance and active transport by TGF-β 1 in alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 285: L1192–L1200, 2003 [DOI] [PubMed] [Google Scholar]
- 141. Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10: R130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wilson SM, Brown SG, McTavish N, McNeill RP, Husband EM, Inglis SK, Olver RE, Clunes MT. Expression of intermediate-conductance, Ca2+-activated K+ channel (KCNN4) in H441 human distal airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L957–L965, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yamamura H, Ugawa S, Ueda T, Nagao M, Joh T, Shimada S. Epithelial Na+ channel δ subunit is an acid sensor in the human oesophagus. Eur J Pharmacol 600: 32–36, 2008 [DOI] [PubMed] [Google Scholar]
- 144. Yamamura H, Ugawa S, Ueda T, Nagao M, Shimada S. Capsazepine is a novel activator of the δ subunit of the human epithelial Na+ channel. J Biol Chem 279: 44483–44489, 2004 [DOI] [PubMed] [Google Scholar]
- 145. Yamamura H, Ugawa S, Ueda T, Nagao M, Shimada S. Epithelial Na+ channel δ subunit mediates acid-induced ATP release in the human skin. Biochem Biophys Res Commun 373: 155–158, 2008 [DOI] [PubMed] [Google Scholar]
- 146. Yamamura H, Ugawa S, Ueda T, Nagao M, Shimada S. Icilin activates the δ-subunit of the human epithelial Na+ channel. Mol Pharmacol 68: 1142–1147, 2005 [DOI] [PubMed] [Google Scholar]
- 147. Yamamura H, Ugawa S, Ueda T, Nagao M, Shimada S. A novel spliced variant of the epithelial Na+ channel δ-subunit in the human brain. Biochem Biophys Res Commun 349: 317–321, 2006 [DOI] [PubMed] [Google Scholar]
- 148. Yamamura H, Ugawa S, Ueda T, Nagao M, Shimada S. Protons activate the δ-subunit of the epithelial Na+ channel in humans. J Biol Chem 279: 12529–12534, 2004 [DOI] [PubMed] [Google Scholar]
- 149. Yamamura H, Ugawa S, Ueda T, Shimada S. Evans blue is a specific antagonist of the human epithelial Na+ channel δ-subunit. J Pharmacol Exp Ther 315: 965–969, 2005 [DOI] [PubMed] [Google Scholar]
- 150. Yamamura H, Ugawa S, Ueda T, Shimada S. Expression analysis of the epithelial Na+ channel delta subunit in human melanoma G-361 cells. Biochem Biophys Res Commun 366: 489–492, 2008 [DOI] [PubMed] [Google Scholar]
- 151. Ye X, Acharya R, Herbert JB, Hamilton SE, Folkesson HG. IL-1β stimulates alveolar fluid absorption in fetal guinea pig lungs via the hypothalamus-pituitary-adrenal gland axis. Am J Physiol Lung Cell Mol Physiol 286: L756–L766, 2004 [DOI] [PubMed] [Google Scholar]
- 152. Yue G, Hu P, Oh Y, Jilling T, Shoemaker RL, Benos DJ, Cragoe EJ, Jr, Matalon S. Culture-induced alterations in alveolar type II cell Na+ conductance. Am J Physiol Cell Physiol 265: C630–C640, 1993 [DOI] [PubMed] [Google Scholar]
- 153. Zhang P, Canessa CM. Single channel properties of rat acid-sensitive ion channel-1α, -2a, and -3 expressed in Xenopus oocytes. J Gen Physiol 120: 553–566, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhao KQ, Xiong G, Wilber M, Cohen NA, Kreindler JL. A role for two-pore K+ channels in modulating Na+ absorption and Cl− secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 302: L4–L12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhao RZ, Nie HG, Su XF, Han DY, Lee A, Huang Y, Chang Y, Matalon S, Ji HL. Characterization of a novel splice variant of δ ENaC subunit in human lungs. Am J Physiol Lung Cell Mol Physiol 302: L1262–L1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


