Abstract
Context:
Antisepsis achieved through appropriate use of irrigants is essential for endodontic success. Identification of newer anti-bacterial agents gives alternatives to clean the canal as eradication of the infection prior to obturation does affect prognosis.
Objective:
Comparison of the anti-bacterial action of 3.8% silver diamine fluoride and 2% chlorhexidine gluconate against Enterococcus faecalis in root canals.
Materials and Methods:
Forty-four single-rooted teeth were decoronated, and the root section was enlarged with peeso-reamer (No: 3) to standardize length and diameter. The samples were then autoclaved and divided into two study groups and two control groups. Enterococcus faecalis ATCC 29212 was inoculated into all test samples for 72 hours. The samples were enlarged with peeso-reamer (No: 5) after placement of respective medicament for 24 hours. Shavings were collected and inoculated on Brain Heart Infusion agar for 24 hrs to measure the colony forming units.
Results:
Both 3.8% silver diamine fluoride and 2% chlorhexidine showed a superior capacity to sterilize the root canals than control groups.
Conclusion:
The use of silver diamine fluoride as an endodontic irrigant is feasible as it can effectively remove the microbes present in the canal and circumpulpal dentin.
Keywords: Chlorhexidine, enterococcus faecalis, root canal medicament, silver diamine fluoride
Introduction
With the growing popularity of single visit endodontics, the need of the hour is for anti-bacterial agents that have high effectiveness to remove and prevent any re-growth of micro-organisms. The plethora of contemporary chemicals used for cleaning root canals includes calcium hydroxide, chlorhexidine, phenolic derivatives, iodine-potassium iodide, and sodium hypochlorite.[1] However, Enterococcus faecalis (E. faecalis), a dentinophillic anaerobe, that is commonly associated with failed root canal treatments and recurrent endodontic infection, has shown resistance against most of these medicaments, especially calcium hydroxide.[2,3] Thus, newer medicaments are required to eliminate the endodontic microflora more effectively.
Silver has been around in dentistry since its inception. It has shown good anti-bacterial properties and is used in several areas for disinfection and sterilization.[4–8] Silver diamine fluoride (SDF) is an anti-cariogenic agent, which is deemed to be very effective, especially in pediatric dentistry.[9,10] It has also been used as an adjuvant in endodontic preparation using lasers.[11,12]
Materials and Methods
Forty-four freshly extracted single-rooted teeth were collected for the study. The teeth were decoronated at cemento-enamel junction. Teeth with apices larger than tip of 15 size K file were replaced. The teeth were sectioned at the apical end to standardize the root canal length at 12 mm from cemento-enamel junction. Root canals of all the samples were enlarged with peeso-reamers (No:3) along with 3% sodium hypochlorite and RC Prep™ (premier USA) containing 15% ethylene diamine tetra acetic acid (EDTA). This resulted in cylindrical samples of the root canal with an inner diameter of 1.1 mm and length of 12 mm. The samples were thoroughly washed with 0.9% saline, dried with paper points, and coated with varnish on the outer surface. Apical end was sealed with Cavit G™ (3M ESPE Germany) and stored for 24 hours prior to autoclaving.
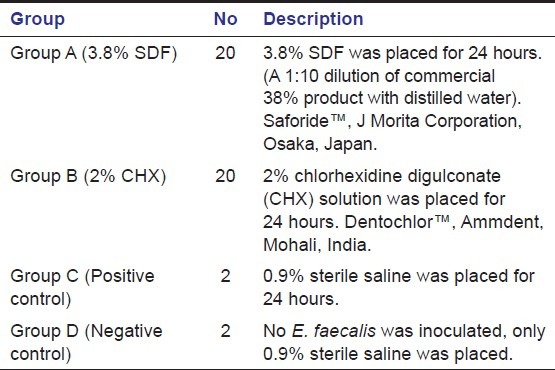
A standardized suspension of E. faecalis (Mc Farland's Standard) was prepared by sub-culturing American Type Cell Culture (ATCC) strain 29212 in Brain Heat Infusion (BHI) broth. All samples except 2 that were used as negative control were individually immersed in 5 milliliters BHI broth containing 1 milliliter of bacterial suspension. The 42 inoculated teeth were stored at 37°C and 100% humidity for 72 hours to ensure growth of E. faecalis. Forty of these teeth were randomly divided into 2 study groups (A and B), the remaining 2 teeth were kept as positive control to ensure viability of the organism. [Table 1]
Table 1.
Grouping of the samples for the study
After placement of medicament, the samples were stored for 24 hours at 37°C and 100% humidity. The medicaments were then washed out with sterile saline. A size 5 peeso-reamer (diameter 1.5 mm) was used with 0.9% saline at slow speed to collect shavings from the root canal surface and circumpulpal dentin. These were then inoculated on BHI agar, and the colony-forming units (CFU) were counted after 24 hours.
Result
Culture results from all samples in the test groups A and B showed no colony-forming units at 24 hours. The positive control group showed mean count of 3.8 × 107 CFU, and negative control showed no growth at 24 hours. [Figure 1] The descriptive statistical analysis showed that the two medicaments tested are far superior to the control group. However, since both study groups showed absolutely no growth of E. faecalis, inference statistics was not required.
Figure 1.
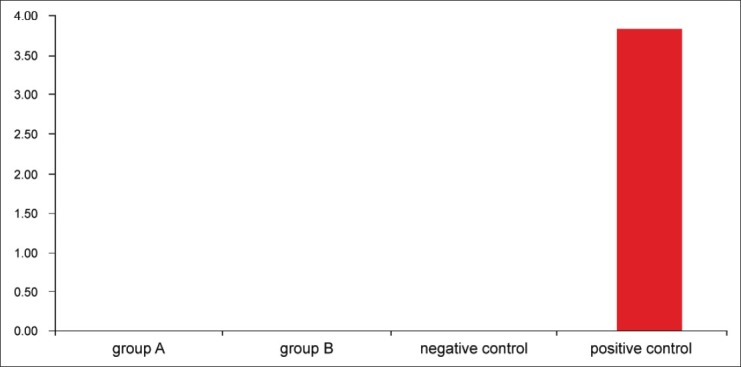
Mean colony forming unit count (× 107) after 24 hours
Discussion
Proficient use of anti-microbials to sterilize the root canal system is a key step to achieve success in contemporary endodontic practice. The rationale is to remove the focus of infection from the radicular system and to ensure aseptic conditions prior to and post-obturation.[1,13,14] The anti-microbial agents available for use both as irrigants and medicaments do not always achieve and maintain sterilization. E. faecalis is a major pathogen associated with chronic recurrent periapical infection and failed endodontic therapy.[15] The ability of this organism to survive is attributed to its resistance against common medicaments like calcium hydroxide and dentinophillic nature, which allows it to permeate up to 400 microns into the dentinal tubules, proliferate, and re-colonize the root canal system leading to failure.[2,3,15]
Silver diamine fluoride (SDF) is an effective anti-cariogenic agent with high fluoride release and capacity to both re-mineralize the tooth surface and increase its surface hardness.[10,11] It has been proposed as a safe, successful, and reasonable caries-preventive agent that meets the criteria of World Health Organization's Millennium goals.[16] However, its anti-bacterial effect against endodontic pathogens has not been explored significantly. Noriko et al. in 2010[17] studied the effect of 3.8% SDF and sodium hypochlorite on in vitro E. faecalis biofilm. They reported 100% efficiency of 3.8% SDF against E. faecalis after a direct 60-minute exposure.
This study compared 3.8% SDF and 2% chlorhexidine gluconate (CHX) as intra-canal medicaments in root canals. 2% CHX is used as both an intra-canal irrigant and medicament that effectively eradicates endodontic microflora.[18] It is considered as a gold standard among root canal medicaments and shows complete eradication of E. faecalis in the root canal.[19,20]
Cylindrical sections of root canals were prepared from coronal and mid portions of the root for symmetry. Apical third often show variations in canal orientation and morphology such that uniformity among the samples would be difficult to achieve. The canals were prepared with a size 3 peeso-reamer in order to enlarge the canal adequately to facilitate both initial growth and penetration of bacteria.
The samples were immersed in standardized suspensions of E. faecalis for 72 hours to ensure good growth and penetration of bacterial biofilm on the root canal surface and into the canals. A size 5 peeso-reamer was used to collect the dentinal shaving so as to remove 400 microns of circumpulpal dentin. This helps to assess that the tested medicaments not only have action in the canal but also penetrate into the dentinal tubules. The evaluation of CFU count from the inoculated debris is an expression of the amount of viable bacteria retrieved from the canal, and this can accurately be used to compare the efficiency of the medicaments.[21]
The results of the study showed that SDF is as effective as 2% CHX in removing E. faecalis from infected root canals. Silver interacts with the sulphydryl and thiol groups present in the bacterial amino acids and nucleic acids.[22,23] This inhibits cell division, cellular respiration, metabolism, and biofilm formation. Several investigators have studied the cellular mechanisms that are affected by silver, thus proving its effectiveness as an anti-bacterial agent.[4,5,24–26]
The advantage of SDF over 2% CHX is that in addition to having similar anti-bacterial properties, the interaction of SDF to teeth results in synergistic formation of fluorapatite.[16] The amount of fluoride release is double of that seen with other fluoridating agents.[27,28] This adds to the anti-bacterial activity and may result in increased longevity and help prevent re-infection in the root canal. The absorption of SDF into dentin also ensures that the dentinophillic bacteria in the circumpulpal dentin are also killed.[17,26] Increase in the surface hardness, decrease in permeability, and increase in fracture resistance of the root canal has all been seen when SDF was initially used as an adjuvant along with lasers in endodontic preparation as it can protect the root dentin from damage due to laser.[11,12,27,28]
Conclusion
This study proves the effectiveness of 3.8% SDF as an intra-canal medicament against E. faecalis. It shows similar anti-microbial activity as 2% CHX. Thus, it can be considered as an alternative medicament to achieve and maintain the sterilization of the root canal and to increase success of root canal treatment.
The mechanical advantages of the use of SDF as an intra-canal medicament and its further action to prevent re-infection of the root canal by preventing biofilm formation and proliferation has to be the focus of future research in this field.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
References
- 1.Law A, Messer H. An evidence based analysis of the antibacterial effectiveness of intracanal medicaments. J Endod. 2004;30:689–94. doi: 10.1097/01.don.0000129959.20011.ee. [DOI] [PubMed] [Google Scholar]
- 2.Chávez de Paz LE, Bergenholtz G, Dahlén G, Svensäter G. Response to alkaline stress by root canal bacteria in biofilms. Int Endod J. 2007;40:344–55. doi: 10.1111/j.1365-2591.2006.01226.x. [DOI] [PubMed] [Google Scholar]
- 3.Brändele N, Zehnder M, Weiger R, Waltimo T. Impact of growth conditions on susceptibility of five microbial species to alkaline stress. J Endod. 2008;34:579–82. doi: 10.1016/j.joen.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Russell AD, Hugo WB. Antimicrobilogical activity and action of silver. Prog Med Chem. 1994;31:351–70. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 5.Lansdow AB. Silver.1: Its antibacterial properties and mechanism of action. J Wound Care. 2002;11:125–30. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 6.Lansdow AB. Silver.2: Toxicity in mammals and how its products aid wound repair. J Wound Care. 2002;11:173–7. doi: 10.12968/jowc.2002.11.5.26398. [DOI] [PubMed] [Google Scholar]
- 7.Lansdow AB. Silver in health care: Antimicrobial effects and safety in use. Curr Probl Dermatol. 2006;33:17–34. doi: 10.1159/000093928. [DOI] [PubMed] [Google Scholar]
- 8.Lansdow AB, Williams A. How safe is silver in wound care? J Wound Care. 2004;13:131–6. doi: 10.12968/jowc.2004.13.4.26596. [DOI] [PubMed] [Google Scholar]
- 9.Yamaga R, Yokomizo I. Arrestment of caries of deciduous teeth with diamine silver fluoride. Dent Outlook. 1969;33:1007–13. [Google Scholar]
- 10.Chu CH, Lo EC, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentinal caries in Chinese pre-school children. J Dent Res. 2002;26:455–7. doi: 10.1177/0810767. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama K, Kimura Y, Matsumoto K, Fujishima A, Miyazaki T. Preventive effect of tooth fracture by pulsed Nd:YAG laser irradiation with diamine silver fluoride solution. J Clin Laser Med Surg. 2001;19:315–8. doi: 10.1089/104454701753342767. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama K, Matsumoto K, Murase J. Permeability of the root canal wall and occlusion of dentinal tubules by Ag(NH3)2 F: A comparison of combined use with pulsed Nd:YAG laser or iontophoresis. J Clin Laser Med Surg. 2000;18:9–14. doi: 10.1089/clm.2000.18.9. [DOI] [PubMed] [Google Scholar]
- 13.Ingle JI, Bakland LK. Endodontics. 5th ed. Hamilton Ontario Canada: Elsevier; 2005. p. 78. [Google Scholar]
- 14.Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–9. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 15.Evans M, Davies JK, Sundqvist G, Figdor V. Mechanisms involved in resistance of enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221–8. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: A caries “Silver fluoride bullet”. J Dent Res. 2009;88:116–25. doi: 10.1177/0022034508329406. [DOI] [PubMed] [Google Scholar]
- 17.Hiraishi N, Yui CK, King NM, Tagami J, Tay FR. Antimicrobial efficacy of 3.8% silver diamine fluoride and its effect on root dentin. J Endod. 2010;36:1026–9. doi: 10.1016/j.joen.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Basrani V, Santos JM, Tjäderhane L, Grad H, Gorduysus O, Huang J, et al. Substantive antimicrobial activity in chlorhexidine treated human dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:240–5. doi: 10.1067/moe.2002.124002. [DOI] [PubMed] [Google Scholar]
- 19.Krithikadatta J, Indira R, Dorothykalyani AL. Disinfection of dentinal tubules with 2% chlorhexidine, 2% metronidazole, bioactive glass when compared with calcium hydroxide as intracanal medicaments. J Endod. 2007;33:1473–6. doi: 10.1016/j.joen.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Vaghela DJ, Kandasamy D, Venkateshbabu N, Jamini N, Arathi G. Disinfection of dentinal tubules with two different formulations of calcium hydroxide as compared to 2% chlorhexidine: As intracanal medicaments against Enterococcus faecalis and Candida albicans: An in vitro study. J Conserv Dent. 2011;14:182–6. doi: 10.4103/0972-0707.82625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavaskar R, de Ataide lde L, Chalakkal P, Pinto MJ, Fernandes KS, Keny RV, et al. An in vitro study comparing the intracanal effectiveness of calcium hydroxide and linezoid based medicaments against Enterococcus faecalis. J Endod. 2012;38:95–100. doi: 10.1016/j.joen.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 22.Wu MY, Suryanarayanan K, van Ooij WJ, Oerther DB. Using microbial genomics to evaluate the effectiveness of silver to prevent biofilm formation. Water Sci Technol. 2007;55:413–9. doi: 10.2166/wst.2007.285. [DOI] [PubMed] [Google Scholar]
- 23.Sacciapoli P, Buxtom D, Rothstein D, Friden P. Antimicrobial activity of silver nitrate against periodontal pathogens. J Periodontal Res. 2001;36:108–13. doi: 10.1034/j.1600-0765.2001.360207.x. [DOI] [PubMed] [Google Scholar]
- 24.Oppermann RV, Rölla G, Johansen JR, Assev S. Thiol groups and reduced acidogenicity of dental plaque in the presence of metal ions in vivo. Scand J Dent Res. 1980;88:389–96. doi: 10.1111/j.1600-0722.1980.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 25.Dibrov P, Dzioba J, Gosnik KK, Häse CC. Cemiosmotic mechanism of antimicrobial activity of Ag(+) in Vibrio cholera. Antimicrob Agents Chemother. 2002;46:2668–70. doi: 10.1128/AAC.46.8.2668-2670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight GM, McIntyre JM, Craig GG, Mulyani, Zilm PS, Gully NJ. Inability to form a biofilm of Streptococcus mutans on silver fluoride and potassium fluoride treated demineralized dentin. Quintessence Int. 2009;40:115–6. [PubMed] [Google Scholar]
- 27.Yamaga R, Nishino M, Yoshida S, Yokomizo I. Diammine silver fluoride and its clinical application. J Osaka Univ Dent Sch. 1972;12:1–20. [PubMed] [Google Scholar]
- 28.Suzuki T, Nishada M, Sobue S, Moriwaki Y. Effects of diamine silver fluoride on tooth enamel. J Osaka Univ Dent Sch. 1974;14:61–72. [PubMed] [Google Scholar]


