Abstract
Objectives
To study the prevalence and risk markers of diabetes mellitus and intermediate hyperglycaemia (IH) in Kisantu, a semirural town in Bas-Congo province, The Democratic Republic of Congo.
Design
A cross-sectional population-based survey.
Settings
A modified WHO STEPwise strategy was used. Capillary glycaemia was measured for fasting plasma glucose and 2-h-postload glucose. Both WHO/IDF (International Diabetes Federation) 2006 and American Diabetes Association (ADA) 2003 diagnostic criteria for diabetes and IH were used.
Participants
1898 subjects aged ⩾ 20 years.
Results
Response rate was 93.7%. Complete data were available for 1759 subjects (86.9%). Crude and standardised (for Doll and UN population) prevalence of diabetes were 4.8% and 4.0–4.2%. Crude IH prevalence was 5.8% (WHO/IDF) and 14.2% (ADA). Independent risk markers for diabetes (p<0.01) were male (OR 2.5), age 50–69 years (OR 2.6), family history (OR 3.5), waist (OR 4.1) and alcohol consumption (OR 0.36). In receiver operating characteristic (ROC) analysis, prediction of diabetes was slightly better by waist than body mass index (BMI). IH defined according to WHO/IDF was associated with BMI (OR 2.6, p<0.001). IH defined according to ADA was associated (p<0.05) with waist (OR 1.4), education level (OR 1.6), BMI (OR 2.4) and physical activity (OR 0.7).
Conclusions
Current prevalence of diabetes in DR Congo exceeds IDF projections for 2030. The lower glucose threshold used by ADA almost triples impaired fasting glucose prevalence compared to WHO/IDF criteria. The high proportion of disorders of glycaemia made up by IH suggests the early stages of a diabetes epidemic.
Keywords: Intermediate Hyperglycaemia; Prevalence; Population Based Survey; Sub-Saharan Africa, Dr Congo
Article summary.
Article focus
We studied the prevalence of diabetes in a semirural town in the Democratic Republic of Congo. Such information is yet not available.
Key-messages
A crude and standardised prevalence of diabetes were 4.8 and 4.0% (Doll's population)—4.2% (UN population).
A rude prevalence of intermediate hyperglycaemia was 5.8% (WHO/IDF definition) and 14.2% (ADA definition).
Risk markers for diabetes were male gender, age, family history, waist and alcohol consumption.
Strengths and limitations
We had to cope with absence of census data and trained field workers.
We had to use (low-cost) capillary blood measures instead of venous samples processed in a laboratory and did not formally validate the test ourselves.
Large group of participants and high participation rate (87%).
Introduction
Sub-Saharan Africa (SSA) has a disproportionate burden of both infectious and chronic diseases.1 The epidemic of non-communicable diseases (NCDs) is rising. Diabetes, an important component of NCDs, has been proposed as a tracer of the other burgeoning chronic diseases because it is well defined, fairly easy to diagnose and common.2
The estimated worldwide prevalence of diabetes among adults was 285 million (6.4%) in 2010, and is predicted to rise to around 438 million (7.7%) by 2030.3 4 For SSA, the number is expected to rise with 98% from 12 million (3.8%) in 2010 to 24 million (4.7%) in 2030. Increase in the prevalence of diabetes is expected to be the largest in developing countries because of population ageing and urbanisation.5
Information on epidemiology of diabetes in SSA has increased of late, but is still limited.1–3 5 High-quality epidemiological studies are difficult to perform in SSA, as they are expensive and labour intensive and populations are often mobile and poorly enumerated.6 International Diabetes Federation (IDF) estimates for many SSA countries are therefore based on extrapolations from distant and probably dissimilar countries and populations. This is also the case for the Democratic Republic of Congo (DRC), where current IDF estimates are derived from two studies in Tanzania, that were published in 1989 and 2000 and that used now-outdated diagnostic criteria.3 7 8 IDF estimates of the national DRC diabetes prevalence of 2.6% in adults, and of 7.4% for impaired glucose tolerance (IGT) should therefore be interpreted with caution.
So far, data on diabetes prevalence in SSA according to current diagnostic criteria are scanty. Few studies have evaluated simultaneously the prevalence of diabetes mellitus and intermediate hyperglycaemia (IH),5 9 and no study has compared the current American Diabetes Association (ADA) and WHO/IDF criteria for impaired fasting glucose (IFG).
The aim of this study is to estimate prevalence rates of diabetes and IH and to determine their main risk markers in the semirural environment of Kisantu, Bas-Congo province, DRC.
Methods
A cross-sectional household survey was conducted in 2007. It was approved by the ethical committee of Kinshasa University (UNIKIN), and informed consent was obtained from all subjects. Design and methods have been described in detail in earlier studies.10
Location
The study was carried out in the four health care areas of Kisantu, a town in Bas-Congo province with approximately 131 000 inhabitants.11 The principal ethnic group is Bakongo (Bantu), mainly the Bantandu subgroup. Farming is the main economic activity.
Study population and sampling procedures
All Congolese people aged 20 or more and living in Kisantu were eligible. Pregnant women and people with serious psychiatric disorders were excluded. The sampling process was implemented in two steps: constitution of the sampling frame preceded actual sampling of subjects. The required sample size was calculated to be 2025 participants.10 Census of inhabited compounds was performed. Sampling in each area was proportionate to its population.11 Given the sampling frame in each area and the intended sample size, a systematic random sample of households was drawn. Sampling was carried out in four stages. All avenues were included, and a systematic (1/2 or 1/3) random sample of compounds was drawn. In each compound, one household was selected randomly. One eligible individual per household was randomly selected. Subjects were asked to come fasting the following morning to the study site. In case of no-show, the interviewer returned the following day. In case of refusal, another individual was selected. The study was preceded by an interviewer training, intensive awareness campaign, and a pilot test in the neighbouring city of Madimba, that was not included in the survey.
Study procedures
Five fixed sites were used for data collection. Subjects went through seven stations (registration, biochemistry, interview, anthropometry and monofilament visual acuity, blood pressure, intraocular pressure and retina results). Capillary glycaemia was measured on the first day in subjects having fasted for ⩾8 h with a Glucocard X-Meter (Menarini Diagnostics Benelux, Zaventem, Belgium), that produces a readout as plasma glucose. On the second day, 2 h postload (75 g glucose, oral glucose tolerance test (oGTT)) glycaemia was measured in subjects who had fasting plasma glucose (FPG) 100–125 mg/dl (5.6–6.9 mmol/l) on day 1, and a second FPG was obtained from subjects with FPG 126–199 mg/dl (7.0–11.1 mmol/l) on the first day.
In agreement with the WHO guidance, and addressing the everyday problems in the field, we used low-technology devices for measuring glycaemia. With the Glucocard X device,12 there is no influence of haematocrite on glucose values within the normoglycaemia range, which is important in screening situations as during our study period. Testing showed that reproducibility is good; the device is robust, easy to use and without the need of extensive training.
Diagnostic criteria
ADA 200313 and WHO/IDF 2006 criteria14 were used to define new cases of diabetes: FPG ⩾7.0 mmol/l, which was confirmed by repeat testing unless unequivocal hyperglycaemia (FPG ⩾11.1 mmol/l) was present, or 2 h plasma glucose ⩾11.1 mmol/l during oGTT. IGT was defined as FPG<7.0 mmol/l, and 2 h plasma glucose ⩾7.8 and <11.1 mmol/l during oGTT. IFG was defined as FPG 6.1–6.9 mmol/l, and 2 h plasma glucose <7.8 mmol/l during oGTT, or as subjects in whom a provisional diagnosis of diabetes based on an initial FPG 7.0–11.1 mmol/l was not confirmed on repeat testing (second FPG 6.1–6.9 mmol/l). ADA 2003 diagnostic criteria for IFG (FPG 5.6–6.9 mmol/l) were used in a secondary analysis. IH refers to subjects with IGT and/or IFG.
Hypertension was defined as an average of two blood-pressure measurements ⩾140/90 mm Hg,10 obesity as body mass index (BMI) ⩾30 kg/m2 and abdominal obesity as waist circumference ⩾94 cm in men and ⩾80 cm in women.15 Sedentary lifestyle was defined with respect to occupation.10 Family history of diabetes mellitus was defined as diabetes in first-degree relatives. Alcohol consumption was classified as current consumption or not, marital status as cohabiting or living alone, ethnic origin as Bantandu, other Bakongo or others.
Analyses
Non-responders and responders were compared, as were subjects with complete data and those who were invited but absent on the second day. Continuous data were expressed as averages±SD and categorised data as percentages with 95% CI. Comparisons were made with student t test, χ2 and analysis of variance. A test was considered significant when p<0.05. Crude and age-standardised prevalence for subjects aged ⩾20 years were calculated using both the standard population of Doll et al and UN 2008 population database projections.16 17 Analyses were stratified by gender. SPSS V.16 was used. To study risk markers associated with diabetes and IH, backward stepwise multivariate logistic regression analyses were used. Family history of diabetes, age by group (<50, 50–69 ⩾70 years), gender, marital status, ethnic origin, level of education, physical activity, alcohol consumption and either obesity or abdominal obesity, and all interactions with a family history were included in the initial model. Receiver-operating characteristic (ROC) analysis compared performance of BMI and waist size to predict diabetes risk.
Results
Study population
The sample consisted of 2025 selected subjects. The response rate was 93.7% (1898/2025). Complete data were available for 1759 subjects (86.9%), as two subjects were aged <20 years, FPG was not available in 30 subjects on day one, and 107 did not repeat tests on day two (figure 1).
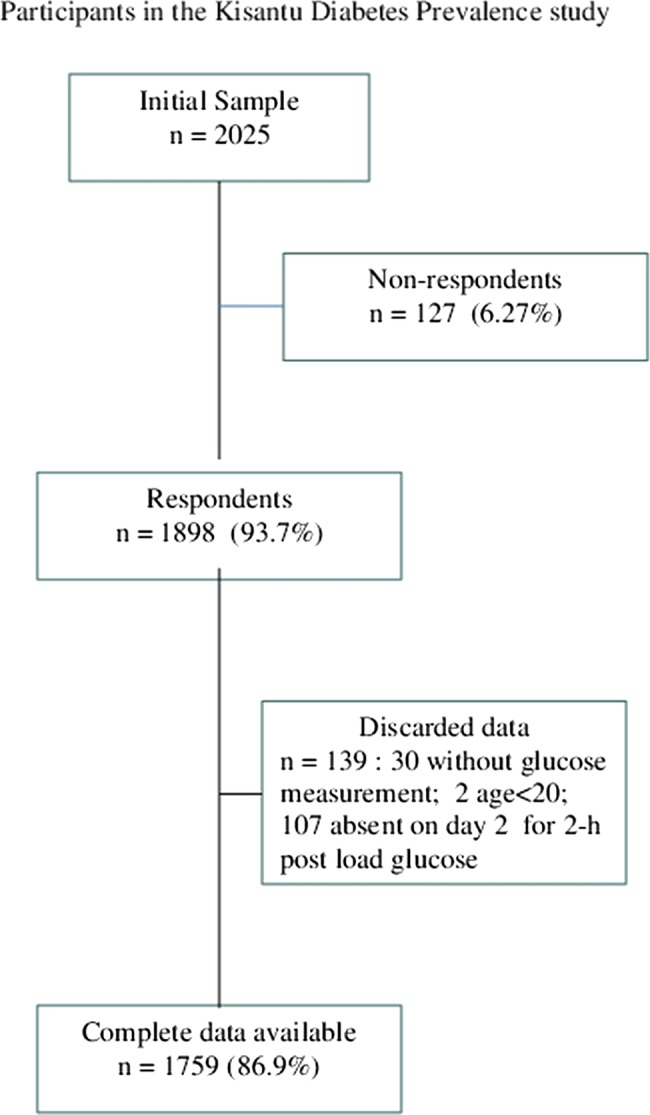
Figure 1.
Characteristics of participants
The average age of 1759 subjects with complete data was 51.5±16.4 years, and 960 (54.6%) were aged >50 years. Women represented 64.1% (n=1128; p<0.001) of subjects. Mean BMI and waist circumference were 21.7±4.6 kg/m² and 79.1±12.7 cm, respectively. Both BMI and waist size were higher in women (p<0.001 for both table 1). Obesity was found in 6.4%, and more prevalent in women (8.6% vs 2.6%). Among subjects with BMI<25 kg/m², 2.9% of men and 27.0% of women had abdominal obesity. Hypertension prevalence was 9.8%.
Table 1 .
Clinical characteristics and crude prevalence of diabetes, intermediate hyperglycaemia or normoglycaemia according to WHO/IDF 2006 criteria13 or ADA 2003 criteria12 of 1759 adults in Kisantu, DRCongo.
| WHO/ADA |
ADA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Diabetes | Intermediate hyperglycaemia | Normal | p Value* | Diabetes | Intermediate hyperglycaemia | Normal | p Value* | |||
| N | 1759 | 85 | 102 | 1572 | 85 | 250 | 1424 | ||||
| Prevalence | 4.8% | 5.8% | 89.4% | 4.8% | 14.2% | 81.0% | |||||
| 95% CI | 3.8–5.9 | 4.8–7.0 | 87.9–90.8 | 3.8–5.9 | 12.6–15.8 | 79.1–82.8 | |||||
| Men (n=631) | 5.5% | 4.6% | 89.9% | 5.5% | 12.0% | 82.4% | |||||
| Women (n=1128) | 4.4% | 6.5% | 89.1% | 4.4% | 15.4% | 80.2% | |||||
| Age (years) | |||||||||||
| Men | 53.0±17.0 | 56.6±15.2 | 56.0±15.9 | 52.6±17.2 | 0.26 | 56.6±15.2 | 52.5±15.7 | 52.9±17.3 | 0.40 | ||
| Women | 50.7±16.0 | 55.2±15.2 | 50.0±16.3 | 50.5±16.0 | 0.13 | 55.2±15.2 | 50.9±14.9 | 50.4±16.2 | 0.12 | ||
| BMI (kg/m2) | |||||||||||
| Men | 20.6±3.5 | 21.7±4.7 | 21.5±4.4 | 20.5±3.4 | 0.06 | 21.7±4.6 | 21.6±4.2 | 20.4±3.3 | 0.03 | ||
| Women | 22.3±4.9 | 24.8±5.7 | 23.1±5.6 | 22.1±4.8 | 0.001 | 24.7±5.7 | 22.8±5.3 | 22.1±4.8 | 0.001 | ||
| Waist (cm) | |||||||||||
| Men | 77.5±11.0 | 84.4±14.2 | 80.5±13.5 | 76.9±10.5 | 0.001 | 84.4±14.3 | 80.7±13.3 | 76.5±0.1 | 0.001 | ||
| Women | 80.1±13.5 | 88.5±13.7 | 81.4±13.2 | 79.5±13.3 | 0.001 | 88.5±13.8 | 80.5±13.0 | 79.5±13.4 | 0.0001 | ||
| Blood pressure (mm Hg) Systolic | |||||||||||
| Men | 124.4±24.1 | 131.9±25.0 | 130.5±19.0 | 123.6±21.3 | 0.03 | 131.9±24.6 | 125.2±18.9 | 123.8±21.6 | 0.09 | ||
| Women | 119.2±22.3 | 133.1±28.4 | 126.2±21.2 | 120.1±21.0 | 0.001 | 133.9±30.9 | 124.4±22.9 | 117.4±21.2 | 0.001 | ||
| Diastolic | |||||||||||
| Men | 76.9±12.4 | 81.2±12.7 | 80.7±12.3 | 76.4±12.3 | 0.02 | 81.2±12.7 | 77.1±13.0 | 76.5±12.3 | 0.09 | ||
| Women | 73.9±12.4 | 80.7±13.3 | 77.9±12.6 | 74.5±12.3 | 0.001 | 80.4±13.8 | 75.9±12.1 | 73.2±12.2 | 0.001 | ||
| Glycaemia (mmol/l) | |||||||||||
| Men | 5.4±2.5 | 12.6±7.2 | 6.4±0.6 | 4.9±0.5 | 0.001 | 12.6±7.2 | 6.0±0.5 | 4.8±0.5 | 0.001 | ||
| Women | 5.2±1.5 | 9.9±4.6 | 6.5±0.4 | 5.0±0.5 | 0.001 | 9.9±4.6 | 6.1±0.5 | 4.8±0.5 | 0.001 | ||
ADA, American Diabetes Association; BMI, body mass index; IDF, International Diabetes Federation.
*p Values relate to the degree of significance when comparing the means between diabetes, IH and normal and are based on the analysis of variance.
Most participants (n=1078; 61.3%) were of Bantandu ethnicity, and 82.3% had a secondary education level (n=1448). Many participants were farmers or animal breeders (49.1%; n=864), yet only 31.4% (n=553) walked ⩾1 h every day.
Incomplete censuses and the absence of key demographic data in the Kisantu area have precluded comparison of study participant characteristics with those in the general population.
Characteristics of non-responders and second-day absentees
Non-responders (n=107) were younger than responders (39.6±12.0 vs 51.5±16.4 years; p<0.001), and were more frequently male (53.2 vs 35.9%; p<0.001). Age, gender, BMI, waist size and systolic and diastolic blood pressures were not significantly different in second-day absentees and in participants with complete data. The first-day FPG, as expected, was significantly higher in second-day absentees (6.1±0.5 vs 5.3±1.9 mmol/l; p<0.05).
Prevalence of diabetes mellitus
Crude prevalence of diabetes mellitus was 4.8% (95% CI 3.9% to 5.9%; n=85) (table 1): men 5.5% (95% CI 3.9% to 7.6%) and women 4.4% (95% CI 3.3% to 5.8%). Standardised prevalence was 4.2% and 4.0% according to Doll et al and UN world population prospects, respectively.
People with previously diagnosed diabetes represented 3.4% (n=59) of all subjects, and 69.4% of diabetes cases. Use of oGTT added only a single case of newly diagnosed diabetes. All other newly diagnosed cases were, therefore, detected using FPG. The highest diabetes prevalence was observed in the 50–69 age group (n=49).
Prevalence of intermediate hyperglycaemia
According to WHO/IDF criteria, crude prevalence of IFG, IGT and IH was 4.7% (n=82), 1.1% (n=20) and 5.8% (95% CI 4.8% to 7.0%; n=102), respectively. IH was observed in 4.6% of men and 6.5% of women (table 1). IH was frequently observed in younger age groups: n=40 (6.4%) in age group 30–49 vs n=33 (4.6%) in age group 50–69.
According to ADA 2003 criteria, IH prevalence was 14.2% (95% CI 12.6% to 15.8%; n=250): men 12.0%and women 15.4%. IFG was present in 12.6% (n=221) of subjects, and IGT in 1.6% (n=29).
Standardised IH prevalence according to Doll et al was 5.5% (WHO/IDF criteria) and 13.8% (ADA criteria). According to UN world population prospects, it was 5.7% (WHO/IDF) (men 3.9%, women 6.6%) and 13.7% (ADA) (men 12.6%, women 15.4%).
Prevalence of disorders of glycaemia
According to WHO/IDF criteria 10.6% (95% CI 9.2% to 12.1%) of study participants had a disorder of glycaemia, that is, diabetes plus IH (men 10.1%; women 10.8%). Based on ADA criteria, disorders of glycaemia were observed in 19% of subjects (95% CI 17.2% to 20.9%; men 17.5% and women 19.8%). The proportion of disorders of glycaemia made up by IH was 54.7% and 74.7% according to WDF/IDF and ADA criteria, respectively.
A bivariate analysis of risk markers
Age was not significantly different in subjects with diabetes, IH or normoglycaemia. Increasing degrees of glucose intolerance were associated with higher BMI values in both sexes, although average BMI values were within the normal range in all categories. Higher waist circumferences were observed in both sexes in subjects with diabetes and IH. Similarly, systolic and diastolic blood pressures were found to be increased in subjects with IH or diabetes (table 1).
The current alcohol use was less prevalent in subjects with diabetes or IH compared to normoglycaemic people (p<0.05). Family history of diabetes (%) was higher in people with diabetes (36.5%) than in IH (12.7%) and normoglycaemics (8.2%, p<0.001). No statistical differences were observed between the three groups in terms of marital status, ethnicity and level of education or occupation.
Multivariate analyses of risk markers
Independent risk markers for diabetes were male (OR 2.5; 95% CI 1.4% to 4.2%), age 50–69 years (OR 2.6; 95% CI 1.5% to 4.3%), family history of diabetes (OR 3.5; 95% CI 1.8% to 6.8%) and waist circumference (OR 4.1; 95% CI 2.5 %to 6.8%). If family history was negative, alcohol consumption was protective (OR 0.4; 95% CI 0.2v to 0.7%). If family history was positive, alcohol consumption was a risk marker (OR=3.8; 95% CI 1.4% to 10.7%). P for interaction: 0.01.
A similar model that compared IH according to WHO/IDF criteria with normal people resulted in only one risk marker: obesity (OR 2.6; 95% CI 1.4% to 4.6%). IH defined according to ADA was associated with waist circumference (OR 1,4; 95% CI 1.1% to 1.9%) and primary education level (OR 1.6; 95% CI 1.1% to 2.6%). Moderate physical activity was protective. BMI predicted IH (OR 0.7; 95% CI 0.5% to 0.9%), when it replaced waist circumference in the model.
An ROC analysis
Upon an ROC analysis, waist circumference (AUC 0.68; 95% CI 0.62% to 0.74%) predicted diabetes slightly better than BMI (AUC 0.61; 95% CI 0.54% to 0.68%). The difference in AUC was 0.08; 95% CI 0.03% to 0.11%.
Discussion
This analytical cross-sectional survey with a participation rate of 87% proves that prevalence studies on NCDs are feasible, even in a Central African country which is one of the poorest countries in the world.
Crude prevalence of diabetes in adults in this study (4.8%) is higher than 2009 IDF estimates for DRC (2.6% for crude national prevalence; 3.2% prevalence adjusted to world population), and already exceeds 2030 IDF projections (3.2–4.4%).3 4 The present data from DRC are, however, largely in line with recent results from other countries in the region3 9 18 and from the Kivu region.19 The standardised prevalence in our study is slightly lower than the crude prevalence (4.0–4.2% vs 4.8%). Other countries in SSA show similar results.3
The crude prevalence of the present survey may have been influenced by the structure of the study sample that contained relatively few individuals aged <50 years in whom diabetes tends to be less prevalent. In the present study, people aged <20 years have been excluded, meaning that our population structure was truncated. The under-representation of young adult men in our study is not unique in SSA: women represented 52, 58, 62 and even 79% of study participants in previous studies.20–22 This may, in part, be due to young men migrating to larger cities or mining areas for economic reasons. As agricultural activities are the main occupation of the Kisantu population, some men may also have preferred to go out to work in the fields. As young men tend to be less obese, this under-representation may influence the crude, but not the standardised, prevalence rate of diabetes and IH.
Contrary to most other studies in SSA, newly detected diabetes was low (31%) in our study. Except in urban South Africa and in one study in rural Sudan, fewer than 50% of participants in previous surveys knew they had diabetes.5 This may reflect effects of repeated diabetes awareness campaigns and level of diabetes care in Kisantu.
Crude prevalence of IH in the present survey (5.8 and 14.2% according to WHO/IDF and ADA criteria, respectively) reflects mainly the proportion of subjects with IFG. The lower glucose threshold used by ADA almost triples IFG prevalence compared to WHO/IDF criteria, as was previously observed in surveys in Denmark, India and the USA.14 As a consequence, disorders of glycaemia, that is, diabetes plus IH, according to ADA criteria are observed in 19.0% of the semirural adult population of Kisantu. Together with the high proportion of disorders of glycaemia made up by IH (54.7 and 74.7% according to WHO/IDF and ADA, respectively), these data suggest the early stages of a diabetes epidemic in DRC, probably more in urban than in rural communities.19 It is noteworthy that these observations are made in people with normal average BMI values, according to Caucasian criteria, a finding that also has been reported elsewhere in SSA.19
A cross-sectional study can only prove an association between risk markers and disease, not a cause-and-effect relationship. However, combining statistical association and clinical reasoning may provide an indication of such an effect, even in the absence of a longitudinal study. Here, we use the term risk marker for a variable with both a significant independent association with diabetes and a clinical suggestion about the direction of this association. Independent risk markers for diabetes observed in this survey are similar to those identified elsewhere in SSA: male gender, age 50–59 years, family history and waist circumference. BMI and waist circumference were risk markers for IH, while moderate physical activity level was a protective factor. The latter observation supports the conclusions of a recent study in Cameroon23: modest population-based changes in physical activity may have significant benefits in terms of reducing the emerging burden of metabolic diseases.
We identified a percentage of known diabetes which is higher than that found elsewhere.24 This may result from a well-organised diabetes programme including awareness campaigns in the past. Of course, this does not influence the prevalence rates we report.
Main challenges for this study were the absence of reliable census data and trained field workers, the difficulty of motivating the population, and performing biochemical tests using low-cost, robust and simple technology. As in similar situations elsewhere, we had to rely on cluster sampling.25 26 Basic principles of the WHO STEPwise method were followed,25 but we added repeat FPG testing or 2 h postload glucose in prespecified subgroups. This is consistent with WHO/IDF and ADA recommendations to enhance chances of detecting diabetes cases and differentiate between IFG and IGT.13–14 27 As 2 h postload glucose was carried out in only a subset of participants, the prevalence of diabetes and IGT may have been underestimated. However, most characteristics are perfectly comparable between attenders and non-attenders, but non-attenders tended to be younger—and therefore related to even less diabetes risk compared to attenders—and mostly males, unable to attend because they were working in the fields or far from their home town—and therefore unlikely to have an undetected diabetes. If none of the non-attenders would have diabetes, the prevalence would decrease from 4.8 to 4.6%, what in our view has little relevance. We therefore have no reason to expect any important difference between diabetes prevalence in second-day attenders and non-attenders.
Capillary blood glucose was measured because it is easier and less expensive than venous tests. Also, WHO and IDF recognise in under-resourced countries the difficulty of carrying out biochemical tests, unless a low-cost technology is used.14 25 28
We did not perform a validation study of these tests ourselves. However, according to a recent WHO/IDF report,14 fasting values of both methods are identical. Only postprandial values may differ (recommendation 5). With this device, there is no influence of haematocrite on glucose values within the normoglycaemic range, which is important in screening situations as carried out during our study.29 The Glucocard X reports plasma glucose values. Testing showed that reproducibility is good; the device is robust; easy to use and without a need of extensive training.12 Additionally, according to the above mentioned WHO/IDF report, separation of plasma within minutes after sampling is required for obtaining correct values when sampling venous blood. Even sampling in a container with NaF is not sufficient. Systematic separation would be totally impossible in rural RDC.
We have little information about the characteristics of the non-responders. Another weakness is that we were unable to compare our study sample with the general population owing to the lack of an official population census map.
Strengths of our study are the large number of study participants and the high participation rate. This was obtained through visiting non-responders at home after each day of the survey,10 a high level of awareness and strong involvement of community members (health zone, local leaders and churches).
The high prevalence of diabetes mellitus in this rural town, and the even higher prevalence of IDF suggest a large burden of the disease in the region, and let us expect even larger numbers in the future. This indicates a real challenge for the Congolese National diabetes plan.
Supplementary Material
Acknowledgments
The authors thank Okitolonda Wemakoy, Kiloka Nyandwe and Prince Kimpanga of Kinshasa University, School of Public Health, DRC; Mangani Nseka of Kinshasa Univeristy, Teaching Hospital; Marina Devis and Carla Truyers of the Department of General Practice, Catholic University Leuven; and Geert Molenberghs of the Department of Biostatistics. We also acknowledge the field workers, Baka Mampuya, Bungudi Edo, Gitambala Jérémie, Kapambu Laetitia, Kimbeni Fifi, Kuzungulu Bibiche, Luntadila Anita, Mafuta Mamie, Makengo Ange, Makiese Brigitte, Mambingila Richard, Matenda Anita, Mbombo Alpha, Mfutila Espérance, Ndengila Pitchouna, Ngiambila Zimbote, Nseka Baron, Nsiala Floribert, Nsoma Malou and Sanga Pélagie.
Footnotes
Contributors: MAM and FB designed the study. MTM, JRM, MM, CBB and DKWK organised and supervised data collection. MTM, MAM and FB performed data analysis. MTM wrote the original paper draft; and MTM, EM and FB contributed to revisions of the paper. All authors contributed to critical revisions of the protocol and manuscript, and approved the final version of the manuscript.
Funding: This work was financed by the 2004–2010 ‘Diabetes in DRC’ project of VLIR-UOS (Flemish Interuniversity Council—University Development Cooperation) and supported by the World Diabetes Foundation.
Competing interests: None.
Ethics approval : UNIKIN (ESP/CE/027).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional unpublished data from the study.
References
- 1.Dalal S, Beunza JJ, Volmink J, et al. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol 2011;40:885–901 [DOI] [PubMed] [Google Scholar]
- 2.Levitt NS. Diabetes in Africa: epidemiology, management and healthcare challenges. Heart 2008;94:1376–82 [DOI] [PubMed] [Google Scholar]
- 3.IDF Diabetes atlas. 4th edn. Brussels, Belgium: International Diabetes Federation, 2009 [Google Scholar]
- 4.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 5.Mbanya JC, Motala AA, Sobngwi E, et al. Diabetes in sub-Saharan Africa. Lancet 2012254–66 [DOI] [PubMed] [Google Scholar]
- 6.Gill GV, Mbanya JC, Ramaiya KL, et al. A sub-Saharan African perspective of diabetes. Diabetologia 2009;52:8–16 [DOI] [PubMed] [Google Scholar]
- 7.McLarty DG, Swai AB, Kitange HM, et al. Prevalence of diabetes and impaired glucose tolerance in rural Tanzania. Lancet 1989;1:871–5 [DOI] [PubMed] [Google Scholar]
- 8.Aspray TJ, Mugusi F, Rashid S, et al. Rural and urban differences in diabetes prevalence in Tanzania: the role of obesity, physical inactivity and urban living. Trans R Soc Trop Med Hyg 2000;94:637–44 [DOI] [PubMed] [Google Scholar]
- 9.Muyer MT, Muls E, Mapatano MA, et al. Le diabète sucré en Afrique sub-Saharienne, une revue systématique de la literature(Diabetes mellitus in sub-Saharan Africa, a systematic review of the literature). Louvain Médical 2008;127:153–65 [Google Scholar]
- 10.Muyer MT, Muls E, Mapatano MA, et al. Estimating prevalence of diabetes in a Congolese town was feasible. J Clin Epidemiol 2011;64:172–81 [DOI] [PubMed] [Google Scholar]
- 11.Kimfuta J.Rapport annuel du Bureau Central de la Zone de Santé de Kisantu (Annual report of the central office of the health zone of Kisantu) 2005.
- 12.American Diabetes Association Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–7 [DOI] [PubMed] [Google Scholar]
- 13.SKUP Glucocard X-meter, Glucocard X-sensor: report from an evaluation organised by SKUP. Scandinavian evaluation of laboratory equipment for primary health care. Bergen: University of Bergen, 2006 [Google Scholar]
- 14.World Health Organization Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation Geneva: WHO, 2006 [Google Scholar]
- 15.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new worldwide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006;23:469–80 [DOI] [PubMed] [Google Scholar]
- 16.Doll R, Waterhouse J, Muir C, et al. Cancer incidence in five continents: a technical report. Geneva, Lyon: International Union against Cancer, International Agency for Research on Cancer, 1966 [Google Scholar]
- 17.United Nations. Department of Economic and Social Affairs. Population Division. World Population Prospects. The 2008 Pevision Population Database. Geneva: United Nations, 2008 [Google Scholar]
- 18.Hwnag CK, Han PV, Zabetian A, et al. Rural diabetes prevalence quintiples over 25 years in low- and middle-income countries: a systematic review and meta-analysis. Diab Res Clin Pract 2012;96:271–85 [DOI] [PubMed] [Google Scholar]
- 19.Katchunga P, Masumbuko B, Belma M, et al. Age and living in an urban environment are major determinants of diabetes among South Kivu Congolese adults. Diabetes Metab 2012;38:324–31 [DOI] [PubMed] [Google Scholar]
- 20.Omar M, Seedat M, Motala A, et al. The prevalence of diabetes mellitus and impaired glucose tolerance in a group of urban South African blacks. S Afr Med J 1993;83:641–3 [PubMed] [Google Scholar]
- 21.Levitt NS, Katzenellenbogen JM, Bradshaw D, et al. The prevalence and identification of risk factors for NIDDM in urban Africans in Cape Town, South Africa. Diabetes Care 1993;16:601–7 [DOI] [PubMed] [Google Scholar]
- 22.Motala A, Esterhuizen T, Gouws E, et al. Diabetes and other disorders of glycemia in a rural South African community: prevalence and associated risk factors. Diabetes Care 2008;31:1783–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assah FK, Ekelund U, Brage S, et al. Urbanization, physical activity, and metabolic health in sub-Saharan Africa. Diabetes Care 2011;34:491–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peer N, Steyn K, Lombard C, et al. Rising diabetes prevalence among urban-dwelling black South-Africans. PLoS One 2012;7: e43336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonita R, deCourten M, Dwyer T, et al. Summary. Monitoring of risk factors for non-communicable diseases: the WHO STEPwise approach. Geneva, Switzerland: World Health Organization, 2001 [Google Scholar]
- 26.Herold J. Surveys and sampling. In: Gregg M. ed. Field epidemiology. Oxford, UK: Oxford University Press, 2008 [Google Scholar]
- 27.American Diabetes Association Position statement. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011:34(Suppl 1):S62–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IDF, Diabetes atlas. 3rd edn Brussels, Belgium: International Diabetes federation, 2006 [Google Scholar]
- 29.Arens S, Moons V, Meuleman P, et al. Evaluation of Glucocard Memory 2 and Acctrend sensor blood glucose meters. Clin Chem Lab Med 1998;36:47–52 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


