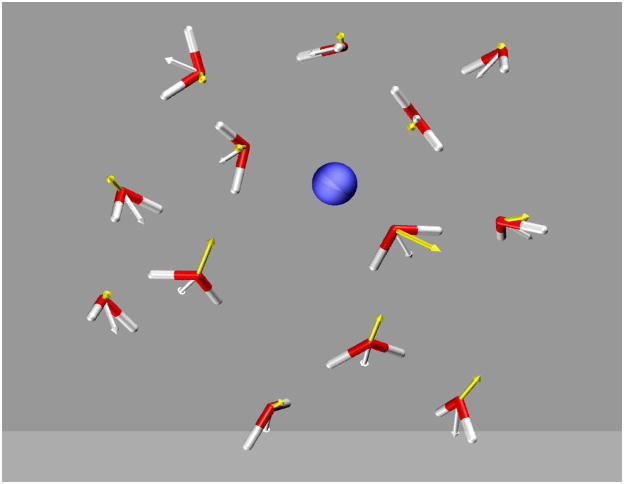
Figure 2.
The reorientation and polarization response of water upon the insertion of a cation (K+) into the bulk water. The yellow vector on each water molecule represents the net induced dipole moment because of the electric field of the ion and other water molecules. The white vector is the permanent (gas-phase) dipole moments (1.8 D) of the water molecule. The average dipole moment of a water molecule in liquid is 2.6–3.0 D according to various theoretical calculations. The snapshot is taken from molecular dynamics simulations of K+ in water using the AMOEBA potential (Ren & Ponder, 2003).