Figure 1. Ca2+ cycling in cardiomyocytes and regulation by PKA.
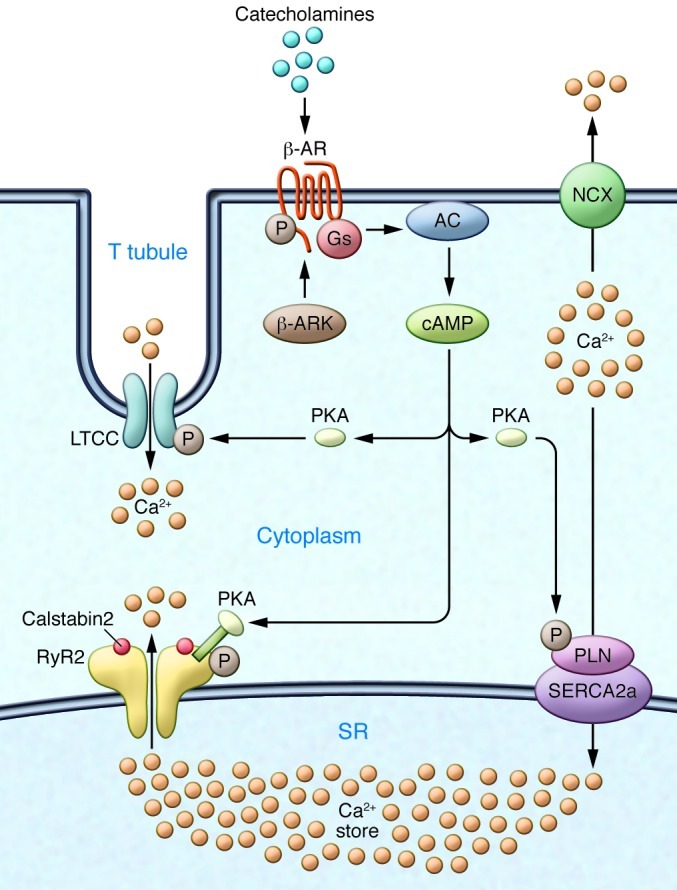
EC coupling in the heart starts with depolarization of the T tubule, which activates voltage-gated L-type Ca2+ channels (LTCCs) in the plasma membrane. Ca2+ influx via LTCCs triggers Ca2+ release from the SR via RyR2 (SR Ca2+ release channel). During systole, the free intracellular Ca2+ concentration increases ten-fold from ∼100 nM to ∼1 μM, which enables muscle contraction. The β-AR signaling pathway can increase the Ca2+ transient by activating the trigger (LTCC), release (RyR2), and uptake (SERCA/phospholamban [SERCA/PLN]) pathways. Catecholamine activation of β-ARs allows for the activation of adenylate cyclase (AC), mediated by specific G proteins (Gs), and the generation of cAMP, which in turn activates PKA. Relaxation occurs after intracellular Ca2+ is pumped out of the cytoplasm by SERCA2a, which is regulated by phospholamban. In addition, Ca2+ is extruded from the cell by the sarcolemmal NCX. RyR2 is a macromolecular complex comprised of four RyR2 monomers, PP1-spinophilin, PP2A-PR130, PKA-PDE4D3-mAKAP, calstabin2, CaMKII, and calmodulin. Calsequestrin regulates luminal SR free Ca2+, and junctin and triadin help maintain the integrity of the T tubule–SR junction. β-ARK, β-AR kinase.
