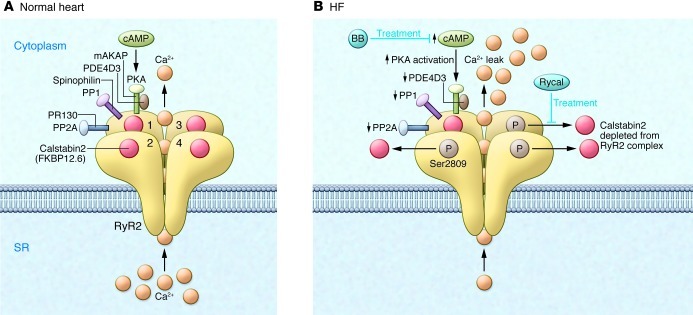
Figure 3. Dysfunctional RyR2 in failing hearts.
(A) The RyR2 macromolecular complex includes four identical RyR2 subunits (numerals 1–4 indicate the four monomers). Each RyR2 subunit binds one calstabin2 (also known as FKBP12.6) as well as mAKAP, to which PKA catalytic and regulatory subunits and PDE4D3 are bound; PP2A and its targeting protein PR130; and PP1 and its targeting protein sphinophilin (accessory molecules are only shown for one of the four RyR2 subunits, except calstabin2, which is shown for all four RyR2 subunits). The β-adrenergic signaling pathway can activate PKA through the second messenger cAMP. (B) In HF, PKA hyperphosphorylation of Ser2809, due to reduced PDE4D3, PP1, and PP2A levels in the RyR2 macromolecular complex, depletes calstabin2 from the RyR2 channel complex. The functional effect of these changes in the macromolecular composition of RyR2 is a pathological increase in Ca2+-dependent activation of RyR2 and depletion of SR Ca2+ stores, as well as functional uncoupling of RyR2 from their neighboring channels. Ca2+ leak due to abnormal RyR2 channel openings may be prevented by treatment with β-blockers (BBs), which interfere with the upstream β-AR signaling pathway, or with Rycals, which selectively increase the binding affinity of calstabin2 to PKA-phosphorylated and oxidized RyR2.