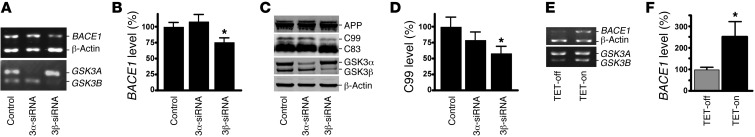
Figure 2. GSK3β, but not GSK3α, regulates BACE1 gene expression and APP processing.
(A) SH-SY5Y human neuroblastoma cells were transfected with scrambled GSK3α or GSK3β isoform–specific siRNA. RNA was extracted, and semiquantitative RT-PCR was performed to measure endogenous human BACE1, GSK3A, GSK3B, and β-actin mRNA levels with specific primers recognizing the coding sequence of each gene. PCR products after 28 cycles were analyzed on 1.2% agarose gel. (B) Endogenous BACE1 mRNA was significantly reduced with GSK3β, but not GSK3α, isoform–specific knockdown. The values are expressed as mean ± SEM. n = 3; *P < 0.05, Student’s t test. (C) 20E2 cells were transfected with scrambled or GSK3α, or GSK3β isoform–specific siRNA while cotreated with L685,458 to block γ-secretase activity. Full-length APP and CTF fragments were detected with C20 antibody. GSK3α and GSK3β were detected using a monoclonal GSK3α/β antibody. GSK3α and GSK3β isoforms were selectively reduced by the isoform-specific siRNA. β-Actin served as an internal control and was detected using a monoclonal anti–β-actin antibody, AC-15. (D) GSK3β-specific knockdown significantly reduced C99 levels. GSK3α-specific knockdown did not have any significant effect. The values are expressed as mean ± SEM. n = 4; *P < 0.05, Student’s t test. (E) Tetracycline-regulated SHSY5Y cells were induced to express constitutively active S9A-GSK3β. Endogenous human BACE1 mRNA levels were assessed as described above. Tetracyline-induced S9A-GSK3β significantly increased BACE1 expression. (F) Quantification of the endogenous BACE1 mRNA level. Values are expressed as mean ± SEM. n = 4; *P < 0.05, Student’s t test.