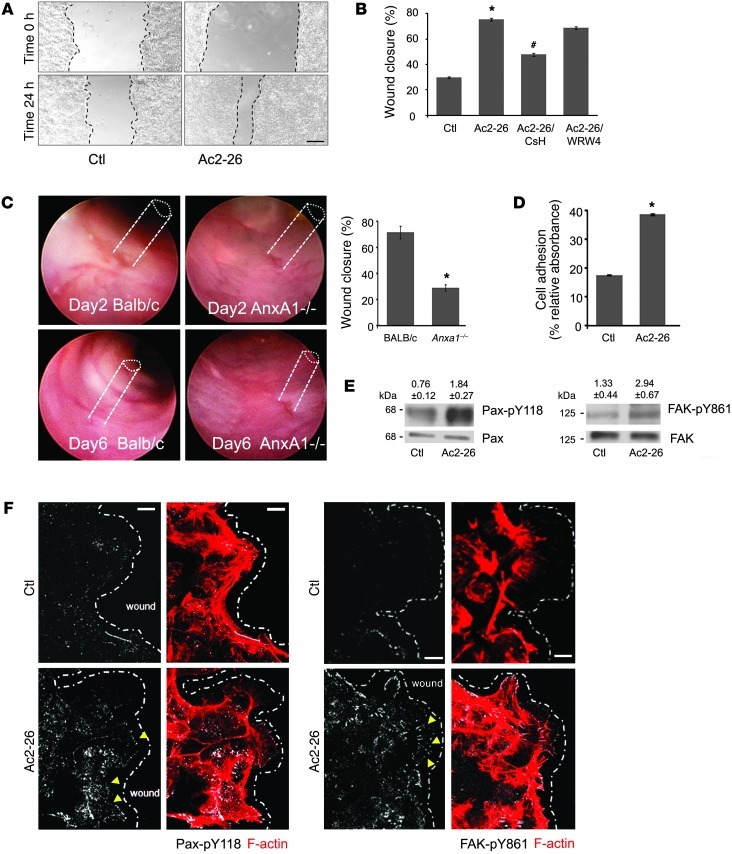
Figure 1. ANXA1 regulates wound healing via FPR1 signaling in IECs and stimulates phosphorylation of focal adhesion proteins (FAK and Pax).
(A) SK-CO15 monolayers were subjected to scratch wound assay in the presence of Ac2-26 peptide (3 μM). Wound widths were determined at 0 and 24 hours. Photomicrograph shows representative results for control (Ctl) and Ac2-26–treated cells. Scale bar: 200 μm. (B) SK-CO15 cells were also incubated with Ac2-26 and CsH (1 μM) or WRW4 (10 μM). The ANXA1 cleavage product Ac2-26 alone significantly enhanced wound closure (*P < 0.0001). The increase in wound closure was inhibited in the presence of CsH (#P < 0.0001) but not WRW4. The experiment was repeated 3 times, and results of 1 representative experiment done with 5 parallel samples are shown. (C) Endoscopic images of colonic mucosal wounds in mice (AnxA1–/– and control BALB/c) at days 2 and 4 after injury. Quantification of wound repair is shown in the graph (mean ± SEM, *P = 0.023, n = 11 mice/group). (D) SK-CO15 cells were plated on ECL gel, and non-adherent cells were removed by washing at 1 hour after plating. Adhesion increased within 15 minutes of Ac2-26 exposure compared with non-stimulated cells (*P < 0.0001). (E) Immunoblot of SK-CO15 cells revealed a significant increase in Pax phosphorylation (Y118, 2.4-fold) and FAK phosphorylation (Y861, 2.2-fold) in cells stimulated with Ac2-26 for 15 minutes compared with unstimulated cells (0.1% DMSO). Normalized signal intensity is indicated above the blots. (F) Laser confocal micrographs of Pax p-Y118 (white) or FAK p-Y861 (white) and F-actin (red) in migrating SK-CO15 cells with or without Ac2-26 (3 μM) for 15 minutes. Photomicrographs are representative of 3 independent experiments done in triplicate. Scale bars: 10 μm.