Abstract
Objective
Prostacyclin and thromboxane mediate opposing cardiovascular actions through receptors termed IP and TP, respectively. When dimerized with IP, the TP shifts to IP-like function. IP localizes to cholesterol-enriched membrane rafts but TP and IPTP heterodimer localization is not defined. We examined these receptors’ membrane localization and the role of rafts in receptor function.
Methods and Results
Microdomain distribution of IP, TP and IPTP heterodimers, was examined in COS-7 cells by measuring energy transfer from renilla luciferase fused receptors to fluorescently labeled rafts. IP raft association was confirmed. TP was raft excluded but redistributed to rafts upon dimerization with IP. Signaling of the IP and IPTP heterodimer, but not TP alone, was suppressed following raft disruption by cholesterol depletion. Cholesterol enrichment also selectively suppressed IP and IPTP function. Native IP and IPTP signaling in smooth muscle cells and macrophages was similarly sensitive to cholesterol manipulation while macrophages from hypercholesterolemic mice displayed suppressed IP and IPTP function.
Conclusions
IP and TP function within distinct microdomains. Raft incorporation of TP in the IPTP heterodimer likely facilitates its signaling shift. We speculate that changes in IP and IPTP signaling following perturbation of membrane cholesterol may contribute to cardiovascular disease associated with hypercholesterolemia.
Keywords: G protein-coupled receptor, dimerization, lipid raft, thromboxane, prostacyclin
Introduction
Prostacyclin (PGI2) and thromboxane (TXA2) are opposing vasoactive mediators generated by the cyclooxygenase (COX) pathway of arachidonic acid metabolism1. PGI2 has established anti-thrombotic, atheroprotective and anti-proliferative actions in vivo2–5. Conversely, TXA2 activates platelets and promotes cell proliferation, migration and adhesion, consistent with its established role in promoting cardiovascular disease (CVD)6. The contribution of TXA2 to CVD is underscored by the established efficacy of low dose aspirin, which inhibits platelet TXA2 biosynthesis, in secondary prevention of stroke and/or heart attack7.
The biological effects of PGI2 and TXA2 are transduced through distinct cell surface G protein-coupled receptors (GPCRs) termed the IP and TP, respectively. The IP is coupled to the Gs-adenylyl cyclase pathway. In humans TP exists as two splice variants, TPα and TPβ. The former TPα is the dominant isoform expressed in most tissues, including vascular smooth muscle cells8 and is the only isoform found in mature platelets9. Multiple G protein pathways lie downstream of the TP although activation of the Gq-phospholipase C and G12/13-Rho pathways appear most relevant to the biological actions of TXA26. We reported physical interaction of the IP and TP to form a heterodimer with a consequent shift in TP function – when dimerized to the IP the TP signals and traffics via IP pathways resulting in an “IP-like” cAMP response to TP agonists, coincident with suppressed TP-Gq signaling10. Importantly these phenomena were evident in vascular smooth muscle cells, which naturally express both receptors, providing a mechanism through which IP can directly limit TP function and protect against CVD.
Plasma membranes are complex, self-organizing structures that dynamically control cell signaling and trafficking. Phase transitions occur at certain cholesterol thresholds giving rise to liquid ordered (Lo) and liquid disordered (Ld) domains11. Lipid rafts, in the Lo domain, are dynamic nanoscale sterol-sphingolipid enriched domains that can coalesce through protein-protein and protein-lipid interactions to form larger more stable platforms12. Multiple membrane proteins, including GPCRs and their downstream signaling molecules, can localize to rafts effectively compartmentalizing signaling events13, 14. Modulation of microdomains can impact signaling of both raft-associated and raft-excluded proteins because of changes in accessibility and proximity of individual signaling components15, 16. Raft signaling is linked with diverse pathologies including CVD focusing interest on factors that modify membrane microdomain function11, 12, 17, 18. Regulation of membrane cholesterol appears critical to control of rafts and associated proteins18, with increasing evidence of a direct effect on signaling and cell function. Depletion of membrane cholesterol is frequently used experimentally to disrupt lipid rafts16, 19, 20, however physiological and pathophysiological cholesterol depletion events are common. Indeed, rafts are a dominant site for cholesterol exchange between cells and lipoproteins regardless of the lipoprotein class21. Oxidized low density lipoproteins, a major cholesterol carrier with established links to atherogenesis, deplete endothelial cells of membrane cholesterol22 while high density lipoproteins, which promote reverse cholesterol transport and are atheroprotective, reduce raft cholesterol in monocytes23. Elevating cholesterol either in vivo or in vitro increases platelet reactivity24, 25 while increased raft formation in hypercholesterolemic mice lead to myeloproliferation and leukocytosis26. Thus, precise control of raft cholesterol content is a critical component of cellular signaling in normal and disease settings. The IP localizes to rafts27 but membrane localization of the TP or the IPTP heterodimer, and the functional consequences for PGI2 and TXA2 interplay, has not been examined. In this study, we explored the role of lipid rafts and cholesterol in IP and TP biology to determine how membrane microdomain homeostasis contributes to PGI2- TXA2 interplay. We confirmed localization and function of the IP within lipid rafts and determined that the TP is predominantly raft excluded. Interestingly, TP’s membrane microdomain localization and function was dramatically altered when the IP and TP dimerized. Our studies provide novel evidence that tight control of membrane cholesterol is essential for the cardiovascular protective signaling of the IP and the restraint it places on TP function.
Methods
Detailed methods are provided in the Supplemental Materials. Receptors were hemagglutinin tagged and fused to either renilla luciferase (rLuc) or yellow fluorescent protein (YFP), as described28. COS-7 cell transfection was with Fugene-6, as described10; experiments were performed 48hrs later. Receptor dimerization was quantified as bioluminescence resonance energy transfer (BRET) from donor (rLUC-receptor) to acceptor (YFP-receptor), as described28. Microdomain localization was defined by energy transfer from rLUC-fused receptor to DiIC16, a fluorescent carbocyanine, that labels the Lo membrane phase16. Second messenger levels were measured using LANCE cAMP and IP-One Tb assay systems.
Results
Membrane domain localization
IP localization to lipid rafts has been reported in transfected and native cells27, consistent with its extensive lipidation29. The absence of lipid modification on the TPα30, 31 predicts Ld distribution. Since these two receptors heterodimerize10, 28, we first examined their individual microdomain distribution.
Fractionation
Cells transfected with either TPα or IP were fractionated under detergent free conditions to separate light (caveolin containing) and heavy (clathrin containing) fractions. Both IP and the TPα were found in the light caveolin-containing fractions (Fig. S1). Such co-segregation with caveolin is often taken as evidence for raft association14, 32. However, determination of raft versus non-raft by cell fractionation, whether in detergent-free conditions or in detergent insoluble isolates, is fraught with technical difficulties and highly dependent on conditions used, often leading to misleading readouts33. Indeed, although it is actually raft excluded in COS-7 cells, the β2- adrenoreceptor (AR) partitions to caveolin-containing fractions in detergent free preparations16. We moved, therefore, to a more direct measure of receptor microdomain localization, referencing β2-AR as raft excluded control.
Membrane labeling with DiIC16
Cells expressing rLUC fused IP or TPα, were loaded with DiIC16. This probe labels the cholesterol rich Lo membrane phase, in which rafts are found, BRET from rLUC to DiIC16 gives a measure of receptor localization to the Lo phase16. Distinct DiIC16 energy transfer curves were seen with IPrLUC and TPrLUC (Fig. 1) - IPrLUC→DiIC16 energy transfer was readily saturable indicating the receptor’s Lo association while the shallow and more linear readout for TPrLUC indicated Lo exclusion. The β2-ARrLUC→DiIC16 curve was also shallow and approached linearity consistent with its reported Lo exclusion in this model16. These data indicate that the TPα is excluded from the Lo phase and that IP and TPα localize to distinct membrane microdomains when expressed separately. We reasoned that to heterodimerize, IP and TPα must organize to co-exist in the same microdomain. We examined whether co-expression of these receptors altered each other’s localization. IPrLUC→DiIC16 energy transfer was not modified by expression of untagged TPα. In the reverse experiment, however, untagged IP shifted TPrLUC→DiIC16 energy transfer to a saturable curve, indistinguishable from the IPrLUC alone (Fig. 1). Thus, upon heterodimerization the IP dominated the TPα causing its redistribution to Lo microdomains.
Fig. 1. IP and TP membrane distribution in living cells.
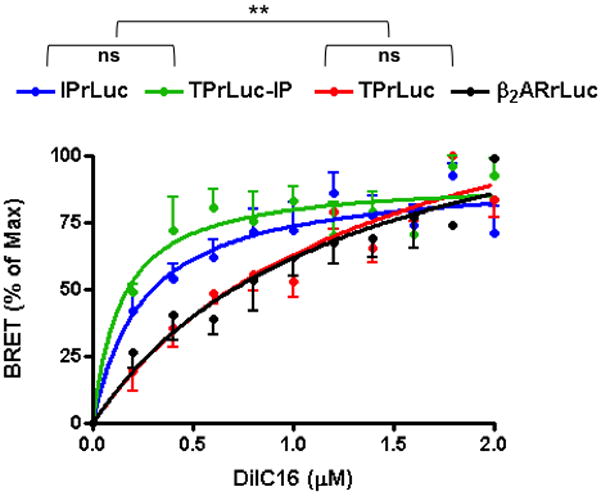
COS-7 cells were transfected with IPrLuc, TPrLuc, TPrLuc plus untagged IP, or β2-ARrLuc and the Lo membrane phase labeled with increasing concentrations of DiIC16. Energy transfer (BRET) from rLuc to DiIC16 was quantified as a measure of the receptor’s localization to the Lo phase. Data are expressed as % of maximum BRET and are mean ± sem; n=3; **p<0.005 for either IPrLUC or TPrLuc-IP versus either TPrLUC or β2-ARrLuc; ns = non significant.
Membrane cholesterol depletion
IP and TP signaling
Acute cholesterol depletion is commonly used to implicate lipid rafts in protein function16, 19, 20. We explored the contribution of Lo domains to IP, TPα or IPTPα function compared with the Lo-excluded β2-AR. Generation of cAMP was used as a read-out of IP or β2-AR function, while InsP generation was a measure of TPα function. TPα switches from InsP to cAMP generation when dimerized with the IP10, therefore TP agonist-induced cAMP was used to assess IPTPα function. Agonists were used at concentrations that maximally activate the receptor in each of the cell models. Cholesterol depletion substantially reduced IP (Fig. 2A) and IPTPα (Fig. 2B) signaling. This contrasts with the raft-excluded β2-AR, in which cAMP generation was elevated (Fig. 2D) reportedly following increased Gs availability upon raft disruption16, and TP- InsP, which was unaltered by cholesterol depletion (Fig. 2C). Thus, in transfected COS- 7 cells, IP and IPTPα function was dependent on raft integrity, further supporting their Lo association, while TP function was not offset by raft disruption, consistent with Lo exclusion. We confirmed that this control mechanism was operational in cells that natively express these receptors. We used primary human aortic smooth muscle cells (hAoSMC) and a macrophage cell line, RAW 264.7. In both cell types, and similar to the COS-7 cells, IP and IPTP coupling to cAMP generation was suppressed by cholesterol depletion (Fig. 2E, F, I and J), while TP-InsP (in hAoSMCs; Fig. 2G) and β2-AR-cAMP signaling (RAW 264.7 cells and hAoSMCs; Fig. 2H, K) was unaltered. A TP-InsP signal was not detected in control or cholesterol depleted RAW 264.7 cells suggesting that, in these cells, the entire population of TP may be heterodimerized with the IP and therefore coupled to the Gs-cAMP cascade.
Fig. 2. Effect of cholesterol depletion on receptor signaling.
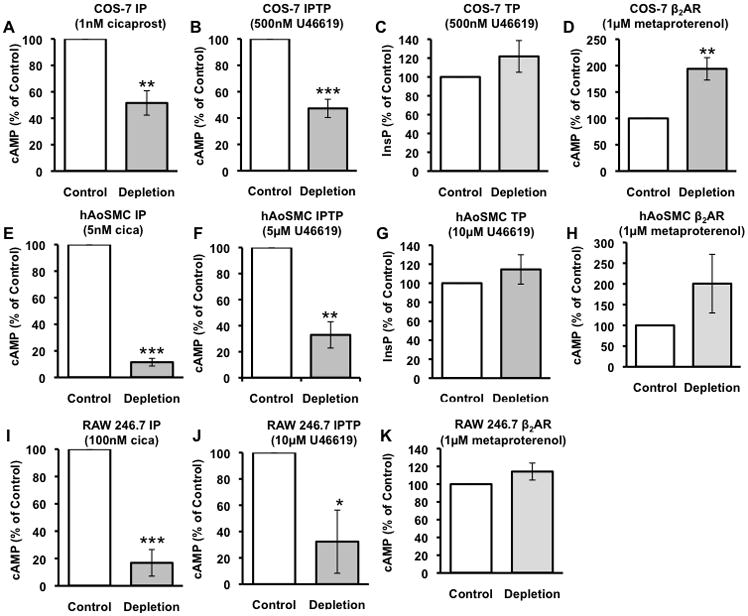
COS-7 cells were transfected with (A) IP alone, (B) IP plus TP, (C) TP alone or (D) β2-AR and, 48hr later, subjected to no treatment (control) or cholesterol depletion (2-hydroxypropyl-β-cyclodextrin 20mM, 1hr). IP and IPTP signaling were determined as cAMP generation in response to cicaprost (IP agonist) or U46619 (TP agonist), respectively; TP signaling was determined as U46619-induced inositol phosphate (InsP) generation and β2-AR signaling as metaproterenol (β2-AR agonist) stimulated cAMP. In (A), (E) and (I) IP signaling is shown for IP-COS-7 cells, hAoSMC and RAW 246.7, respectively. In (B), (F) and (J) IPTP signaling is shown IPTP-COS-7 cells, hAoSMC and RAW 246.7, respectively. In (C) and (G) TP signaling is shown in TP-COS-7 cells and hAoSMC, respectively. In (D), (H) and (K) β2-AR signaling is shown in β2-AR-COS-7 cells hAoSMC and RAW 246.7 respectively. Results are % of control ± sem from n=3. * p<0.05, ** p<0.005, *** p<0.0005 compared to control.
IP and TP dimerization
Studies report that GPCR dimerization is a prerequisite for normal membrane expression and function34–36. Given that our evidence for differential membrane microdomain localization of IP, TPα and IPTPα heterodimers we considered whether raft disruption would modify differentially their physical association. Dimerization was assessed as we described previously28 by measuring BRET from an rLUC-fused receptor to an acceptor YFP-fused receptor. Cholesterol depletion did not alter substantially IP homodimerization, TPα homodimerization or IPTPα heterodimerization (Fig. 3), indicating that Lo-included and -excluded dimers remain associated despite cholesterol depletion. Thus it appears that homo- and hetero- dimerization of the IP and TPα is independent of their microdomain localization.
Fig. 3. IP and TP dimerization in living cells.
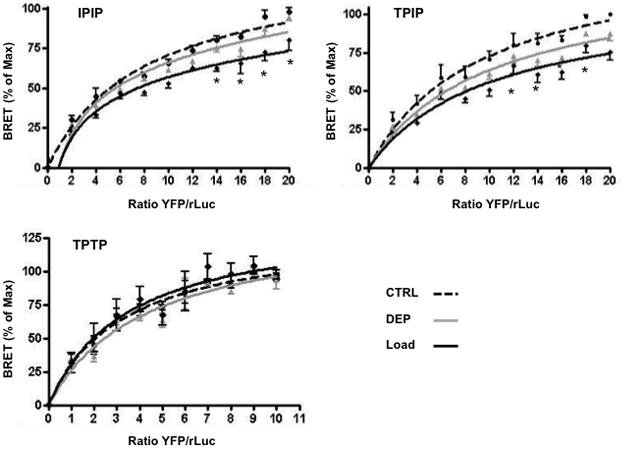
Receptor dimerization was examined by measuring BRET from (A) IPrLuc to IPYFP, (B) TPrLuc to IPYFP or (C) TPrLuc to TP YFP in transfected COS-7 subjected to cholesterol depletion (grey line), loading (solid black line), or no manipulation (dashed line). Data are the % of maximum in control cells vs. the ratio of YFP-receptor/rLuc-receptor and are mean ± sem of n=3.
Effect of Cholesterol Enrichment
IP and TP signaling
The impact of elevating plasma membrane cholesterol on cellular signaling is not well defined although modified raft function and signaling has been reported in cells that are enriched in vitro or in vivo with cholesterol37–39. We explored the effect of cholesterol enrichment on IP, TPα and IPTPα function. Cholesterol loading suppressed cAMP generation in IP and IPTPα transfected cells (Fig. 4A, B). In contrast, signaling of the Lo-excluded β2-AR (cAMP) and TP (InsP) were resistant to cholesterol loading (Fig. 4C, D). Thus, cholesterol enrichment selectively impacted the function of raft-associated GPCRs in the COS-7 model. We confirmed the effect of cholesterol enrichment in both native cells models, hAoSMC and RAW 264.7 cells, demonstrating suppressed IP and IPTP coupling to cAMP generation (Fig. 4E, F, I and J) with unaltered TP-InsP in hAoSMC (Fig. 4G) and β2-AR-cAMP signaling in both native models (Fig. 4H, K). Since hypercholesterolemia is an established risk factor in cardiovascular disease40, 41, we considered whether in vivo elevation of plasma cholesterol would similarly impact selectively IP and IPTP function. Plasma cholesterol levels in low-density lipoprotein receptor deficient (LDLR−/−) mice on normal chow are 3–4 times higher than similarly fed wild type (WT) mice42, coincident with elevated plasma membrane cholesterol content in several cell types43–45. We examined ex vivo IP coupling to cAMP generation in peritoneal macrophages isolated from WT and LDLR−/− mice. Similar to in vitro cholesterol enrichment of transfected or native cells, function of both IP and IPTP was suppressed in macrophages from hypercholesterolemic LDLR−/− mice (Fig. 5).
Fig. 4. Effect of cholesterol enrichment on receptor signaling.
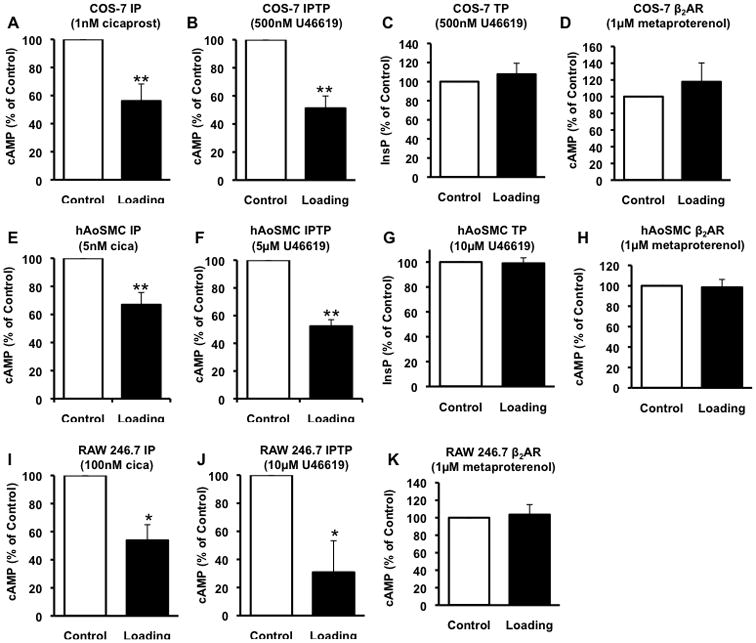
COS-7 cells were transfected with (A) IP alone, (B) IP plus TP, (C) TP alone or (D) β2-AR and, 48hr later, subjected to no treatment (control) or cholesterol loading (cholesterol-methyl-β-cyclodextrin complex 80μg/ml, 1hr). IP and IPTP signaling were determined as cAMP generation in response to cicaprost (IP agonist) or U46619 (TP agonist), respectively; TP signaling was determined as U46619-induced inositol phosphate (InsP) generation and β2-AR signaling as metaproterenol (β2-AR agonist) stimulated cAMP. In (A), (E) and (I) IP signaling is shown for IP-COS-7 cells, hAoSMC and RAW 246.7, respectively. In (B), (F) and (J) IPTP signaling is shown IPTP-COS-7 cells, hAoSMC and RAW 246.7, respectively. In (C) and (G) TP signaling is shown in TP-COS-7 cells and hAoSMC, respectively. In (D), (H) and (K) β2-AR signaling is shown in β2-AR-COS-7 cells hAoSMC and RAW 246.7 respectively. Results are % of control ± sem from n=3. * p<0.05, ** p<0.005 compared to control.
Fig. 5. IP- and IPTP-mediated signaling in mouse peritoneal macrophages.
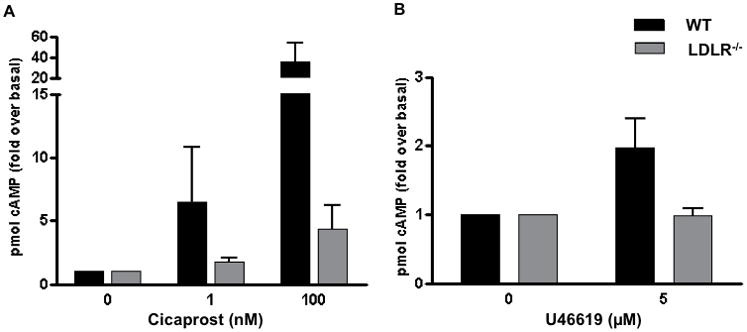
Peritoneal macrophages isolated from wild type (WT) or hypercholesterolemic low density lipoprotein receptor deficient (LDLR−/−) mice (6–8 months of age) were stimulated with (A) cicaprost or (B) U46619 and cAMP generation quantified. Results are fold over basal ± sem; n=6–7; * p<0.05 compared to WT.
IP and TP dimerization
Given that cholesterol enrichment selectively impacted the function of Lo-associated receptors, we examined whether elevated membrane cholesterol modified their dimerization. Dimers remained associated following cholesterol enrichment although, interestingly, a lower BRETmax was seen for the two raft associated species, IPIP and IPTPα), but not the raft excluded TPαTPα (Fig. 3). This differential effect provides further, albeit indirect, evidence that the IPIP and IPTPα reside in a distinct membrane microdomain compared to the TPαTPα.
Receptor surface expression is unaltered by cholesterol enrichement
Cholesterol enrichment can induce sequestration of raft-associated proteins46–48 raising the possibility that reduced IP or IPTP signaling in transfected and native cells exposed to elevated cholesterol simply reflected loss of surface receptor. We examined cell surface expression, by flow cytometry, in the transfected COS-7 model and found no change in IP or TPα, expressed alone or in combination, in control and cholesterol-loaded conditions (Fig. S3A). Similarly, native IP surface expression was not different in peritoneal macrophages from hyper-cholesterolemic mice (either 8 months on a high (42%) fat diet, Fig. S3B or 6 month old LDLR−/− mice on normal chow Fig S3C) compared to normal controls. Thus, suppression of receptor signaling following exposure to high cholesterol, either in vitro or in vivo, does not appear related to loss of cell surface receptor expression.
Discussion
We reported previously a shift of TP signaling from Gq/InsP to a Gs/cAMP IP-like signaling that occurs when IP and TP heterodimerize10. The molecular pathways that control the formation and function of IP and TP homo or heterodimers in co-expressing cells, and the relationship to cardiovascular disease remain, however, poorly understood. Clustering of GPCRs and their downstream signaling proteins in specialized plasma membrane microdomains has emerged as a key feature of cell- and context- specific receptor function13, 14. Little is known about how membrane microdomains influence the formation and function of GPCR dimers. This is particularly interesting when considering heterodimers of receptors like the IP and TP that are distinct in their signaling, cell trafficking and regulation when examined individually1. In this study, we examined the relative localization of IP and TP to Lo membrane phase, and the relationship between these receptors’ respective localization and their dimerization and function.
Lipidation of proteins promotes their association with lipid rafts49. The IP, which is both palmitoylated and isoprenylated29 and is raft localized in this and other27 studies. TPα, which is, in contrast, not lipidated30, 31, was predominantly Lo excluded. Interestingly, upon IP co-expression, redistribution TPα to the Lo phase was evident. IP localization was, however, unaltered by co-expression of the TPα consistent with a dominant effect the IP on TPα membrane microdomain localization. We sought to confirm these findings using a standard methodology of membrane fractionation under detergent-free conditions. However, by this methodology, both IP and TPα partitioned with caveolin- rich fractions (a standard raft marker) with no evidence of their differential localization. Significant concerns have been raised about the reliability of cell fractionation for determination of membrane microdomain localization33, 50. Of particular relevance to our work, detergent-free cell fractionation lacked the resolution to identify exclusion of the β2-AR from rafts while energy transfer to DiIC16, the fluorescent Lo-label that we used, provide clear discrimination receptors that are Lo associated versus Lo excluded16.
Clustering of receptors and their downstream signaling proteins in raft domains appears critical for receptor function13, 14. We next explored whether raft integrity was differentially important for IP and TPα function and dimerization using cholesterol depletion of membrane cholesterol, an established method to disrupt rafts16, 19, 20. As we reported previously, a TXA2 analog U46619 induced a robust cAMP response in IPTPα transfected cells that was similar to the PGI2 analog cicaprost10. The response to either agonist was suppressed following cholesterol depletion of transfected COS-7 cells, as well as in hAoSMC and RAW 246.7, cells that natively co-express the IP and TP. Thus, raft integrity appeared essential to transduction of a Gs-cAMP signal via the IP or the IPTP heterodimer across transfected and native cell models. It may be that cholesterol depletion impacted Gs or adenylyl cyclase function rather than IP or IPTP function per se. However, the increase in cAMP generation through the raft-excluded β2AR that we, and others16, observe argues against a general effect of cholesterol depletion on the Gs-cAMP pathway. In contrast to the IPTP heterodimer, discrete TP signaling to Gq was not affected by cholesterol depletion of TP-transfected COS-7 or hAoSMC. Our data indicate, therefore, that raft disruption by cholesterol depletion can differentially modify signaling of Lo-excluded versus Lo-associated receptors with the former being unaltered or enhanced and the latter being suppressed. Such differential effect of cholesterol depletion consistent with distinct microdomain localization has also been reported for the μ-opioid receptor (both raft and non-raft localized) and δ-opioid receptor (raft excluded)15. Gs is typically localized in rafts and its release from rafts following their disruption16 likely leads to loss of function for raft-associated Gs coupled receptors like the IP and IPTPα heterodimer. Gq, in contrast, is reported to function in both raft and non-raft domains51, consistent with the insensitivity of the TPα-Gq-InsP signal to cholesterol depletion.
To our knowledge, no study has examined the role of rafts or other membrane microdomains in GPCRs dimerization. X-ray crystallographic studies indicate, however, that cholesterol may help to form or stabilize GPCR dimers and that rafts may be critical to these assemblies52. In our model, acute cholesterol depletion did not markedly alter BRET for IP homodimerization, TP homodimerization or IPTPα heterodimerization. This is consistent with a leading model of GPCR dimerization - that dimers are brought to the plasma membrane as a preformed complex assembled in the endoplasmic reticulum (ER)34–36. Indeed, our previous work supports formation of IP and TP containing dimers in the ER28 and is consisted with the current findings that acute disruption of membrane rafts does not modify the dimerization process itself but rather the subsequent function of the receptor complex. Interestingly, when cells were instead subjected to cholesterol enrichment, the BRETmax for IPIP and IPTPα, but not TPTP, was blunted. This may reflect reduced association of the IP and TP to form the heterodimeric species.
However, this is unlikely given that the BRET50, which reflects affinity53, was not altered. It is instead likely that the process of dimer assembly in the ER proceeds normally in cholesterol loaded cells but that elevated cholesterol in the Lo phase suppresses energy transfer between the partners once they reach raft sites. The functional impact of cholesterol loading was clearly evident - signaling via both the IP or IPTP, but not TP or β2-AR, was reduced following cholesterol enrichment, consistent with reports that cholesterol loading can modify raft function37–39.
Plasma cholesterol levels are directly related to cell membrane cholesterol content43–45. Elevated plasma cholesterol, or cholesterol loading of cells in vitro, is linked with increased leukocyte adhesion and inflammation, as well as augmented platelet reactivity and thrombosis24, 25, 54, processes that are restrained by the PGI2-IP- cAMP pathway5, 55. We considered that cholesterol loading of cells in culture may mimic increased plasma cholesterol levels associated with a high fat diet and/or genetic abnormalities. The significant impairment of both the IP and IPTP cAMP signal seen in macrophages from hypercholesterolemic mice supports the notion that elevated cholesterol can modify cell surface receptor function in vivo, and supports the relevance of our in vitro studies to CVD associated with high cholesterol. The mechanism(s) through which cholesterol enrichment modifies cell and receptor function remain ill defined. Cell surface levels of IP and TP were unchanged by cholesterol loading, whether they were expressed alone or in combination, thus it is likely that cholesterol loading functionally modified the IP and IPTP receptor complexes. Enrichment of platelet membranes with cholesterol was reported to decreased membrane fluidity46, 47 and increased rigidity of cholesterol rich membranes can modulate raft-associated signaling48, 56. It may be that the fluid dynamics of the microdomains become less favorable to transduction of the signal from receptor to G protein and/or effector. This possibility is currently under investigation.
The IP is well established to reduce disease severity in a range of cardiovascular disease models5, 55. Further, IP limits the pro-thrombotic and proliferative actions of the TP in vivo55. We determined that formation of an IPTP heterodimer contributes this IP-mediated restraint of TP function10, 28. The importance of GPCR dimerization for physiological and pathophysiological receptor functions is beginning to emerge57–59. We reported a dominant negative influence of a naturally occurring IP mutant (IPR212C) on its wild type counterpart, through dimerization, as a mechanistic basis for the exaggerated loss of platelet responsiveness to PGI2 analogs in platelets from individuals heterozygous for the mutant28. These, and other studies60–62, indicate that understanding GPCR homo- and heterodimerization is critical to determining the function of these receptor pathways in vivo and to their successful therapeutic targeting in human disease.
In summary, our data show that IP localizes and functions in membrane rafts, while TPα is raft excluded but redistributed to rafts when dimerized with the IP. IP function depends on raft integrity as does the modified IP-like function of the TP in an IPTP heterodimer. Thus, we identified lipid rafts as a system used by the cell to partition the signaling and function of the IP and TP based on their homo- or hetero-dimerization and determined the importance of membrane cholesterol homeostasis for normal IP and TP function. The internal consistency of our studies across transfected, primary and ex vivo models demonstrates that membrane cholesterol homeostasis is critical for normal receptor function and least for the receptors that we examined. Coupling of the IP and IPTP to the Gs-cAMP signaling cascade is important for the beneficial actions of PGI2 in cardiovascular disease as well as the restraint placed by the IP on TP’s deleterious cardiovascular actions10, 28, 55. Our studies indicate that elevated plasma cholesterol can significantly suppress this critical signaling pathway across a range of cell types. We speculate that these perturbations in signaling may contribute to altered cardiovascular function in hypercholesterolemic humans.
Supplementary Material
Acknowledgments
We are grateful for the assistance of Ms Jenny Bruce and Ms Victoire Ndong.
Sources of Funding: This work was supported by the National Heart Lung and Blood Institute Grant HL066233 (to E.M.S.) and the American Heart Association Grants 12GRNT11340004 (to E.M.S.) and 09POST2220038 (to S.I.).
Footnotes
Disclosures: None.
References
- 1.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50 (Suppl):S423–428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest. 2006;116:1391–1399. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, Fitzgerald GA. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–1957. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 4.Francois H, Athirakul K, Howell D, Dash R, Mao L, Kim HS, Rockman HA, Fitzgerald GA, Koller BH, Coffman TM. Prostacyclin protects against elevated blood pressure and cardiac fibrosis. Cell Metab. 2005;2:201–207. doi: 10.1016/j.cmet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T, Narumiya S. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004;114:784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther. 2008;118:18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Baigent C, Patrono C. Selective cyclooxygenase 2 inhibitors, aspirin, and cardiovascular disease: a reappraisal. Arthritis Rheum. 2003;48:12–20. doi: 10.1002/art.10738. [DOI] [PubMed] [Google Scholar]
- 8.Kinsella BT. Thromboxane A2 signalling in humans: a ‘Tail’ of two receptors. Biochem Soc Trans. 2001;29:641–654. doi: 10.1042/0300-5127:0290641. [DOI] [PubMed] [Google Scholar]
- 9.Habib A, FitzGerald GA, Maclouf J. Phosphorylation of the thromboxane receptor alpha, the predominant isoform expressed in human platelets. J Biol Chem. 1999;274:2645–2651. doi: 10.1074/jbc.274.5.2645. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SJ, Roche AM, Kostetskaia E, Smyth EM. Dimerization of the human receptors for prostacyclin and thromboxane facilitates thromboxane receptor-mediated cAMP generation. J Biol Chem. 2004;279:53036–53047. doi: 10.1074/jbc.M405002200. [DOI] [PubMed] [Google Scholar]
- 11.Yaqoob P. The nutritional significance of lipid rafts. Annu Rev Nutr. 2009;29:257–282. doi: 10.1146/annurev-nutr-080508-141205. [DOI] [PubMed] [Google Scholar]
- 12.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 13.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 14.Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008;186:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 15.Levitt ES, Clark MJ, Jenkins PM, Martens JR, Traynor JR. Differential effect of membrane cholesterol removal on mu- and delta-opioid receptors: a parallel comparison of acute and chronic signaling to adenylyl cyclase. J Biol Chem. 2009;284:22108–22122. doi: 10.1074/jbc.M109.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pontier SM, Percherancier Y, Galandrin S, Breit A, Gales C, Bouvier M. Cholesterol-dependent separation of the beta2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J Biol Chem. 2008;283:24659–24672. doi: 10.1074/jbc.M800778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chini B, Parenti M. G-protein-coupled receptors, cholesterol and palmitoylation: facts about fats. J Mol Endocrinol. 2009;42:371–379. doi: 10.1677/JME-08-0114. [DOI] [PubMed] [Google Scholar]
- 18.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 19.Jiao X, Zhang N, Xu X, Oppenheim JJ, Jin T. Ligand-induced partitioning of human CXCR1 chemokine receptors with lipid raft microenvironments facilitates G-protein-dependent signaling. Mol Cell Biol. 2005;25:5752–5762. doi: 10.1128/MCB.25.13.5752-5762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouquette-Jazdanian AK, Pelassy C, Breittmayer JP, Aussel C. Revaluation of the role of cholesterol in stabilizing rafts implicated in T cell receptor signaling. Cell Signal. 2006;18:105–122. doi: 10.1016/j.cellsig.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Storey SM, Gallegos AM, Atshaves BP, McIntosh AL, Martin GG, Parr RD, Landrock KK, Kier AB, Ball JM, Schroeder F. Selective cholesterol dynamics between lipoproteins and caveolae/lipid rafts. Biochemistry. 2007;46:13891–13906. doi: 10.1021/bi700690s. [DOI] [PubMed] [Google Scholar]
- 22.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 23.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Ashraf MZ, Podrez EA. Scavenger receptor BI modulates platelet reactivity and thrombosis in dyslipidemia. Blood. 2010;116:1932–1941. doi: 10.1182/blood-2010-02-268508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuart MJ, Gerrard JM, White JG. Effect of cholesterol on production of thromboxane b2 by platelets in vitro. N Engl J Med. 1980;302:6–10. doi: 10.1056/NEJM198001033020102. [DOI] [PubMed] [Google Scholar]
- 26.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Thangavel M, Sun SQ, Kaminsky J, Mahautmr P, Stitham J, Hwa J, Ostrom RS. Adenylyl cyclase type 6 overexpression selectively enhances beta-adrenergic and prostacyclin receptor-mediated inhibition of cardiac fibroblast function because of colocalization in lipid rafts. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:359–369. doi: 10.1007/s00210-007-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim S, Tetruashvily M, Frey AJ, Wilson SJ, Stitham J, Hwa J, Smyth EM. Dominant negative actions of human prostacyclin receptor variant through dimerization: implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2010;30:1802–1809. doi: 10.1161/ATVBAHA.110.208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miggin SM, Lawler OA, Kinsella BT. Palmitoylation of the human prostacyclin receptor. Functional implications of palmitoylation and isoprenylation. J Biol Chem. 2003;278:6947–6958. doi: 10.1074/jbc.M210637200. [DOI] [PubMed] [Google Scholar]
- 30.O’Meara SJ, Kinsella BT. Investigation of the effect of the farnesyl protein transferase inhibitor R115777 on isoprenylation and intracellular signalling by the prostacyclin receptor. Br J Pharmacol. 2004;143:318–330. doi: 10.1038/sj.bjp.0705956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid HM, Kinsella BT. Palmitoylation of the TPbeta isoform of the human thromboxane A2 receptor. Modulation of G protein: effector coupling and modes of receptor internalization. Cell Signal. 2007;19:1056–1070. doi: 10.1016/j.cellsig.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quest AF, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- 33.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- 35.Salahpour A, Angers S, Mercier JF, Lagace M, Marullo S, Bouvier M. Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- 36.Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen DH, Espinoza JC, Taub DD. Cellular cholesterol enrichment impairs T cell activation and chemotaxis. Mech Ageing Dev. 2004;125:641–650. doi: 10.1016/j.mad.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fredrickson DS, Steinberg D. Inhibitors of cholesterol biosynthesis and the problem of hypercholesterolemia. Ann N Y Acad Sci. 1956;64:579–589. doi: 10.1111/j.1749-6632.1956.tb36832.x. [DOI] [PubMed] [Google Scholar]
- 41.LaRosa JC, Hunninghake D, Bush D, Criqui MH, Getz GS, Gotto AM, Jr, Grundy SM, Rakita L, Robertson RM, Weisfeldt ML, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81:1721–1733. doi: 10.1161/01.cir.81.5.1721. [DOI] [PubMed] [Google Scholar]
- 42.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhandaru R, Srinivasan SR, Radhakrisnamurthy B, Berenson GS. Effects of diabetes and high fat-high cholesterol diet on plasma lipid levels and on erythrocyte membrane composition. Atherosclerosis. 1982;42:263–272. doi: 10.1016/0021-9150(82)90156-3. [DOI] [PubMed] [Google Scholar]
- 44.Sun JV, Tepperman HM, Tepperman J. Lipid composition of liver plasma membranes isolated from rats fed a high glucose or a high fat diet. J Nutr. 1979;109:193–201. doi: 10.1093/jn/109.2.193. [DOI] [PubMed] [Google Scholar]
- 45.Shanmugasundaram KR, Visvanathan A, Dhandapani K, Srinivasan N, Rasappan P, Gilbert R, Alladi S, Kancharla S, Vasanthi N. Effect of high-fat diet on cholesterol distribution in plasma lipoproteins, cholesterol esterifying activity in leucocytes, and erythrocyte membrane components studied: importance of body weight. Am J Clin Nutr. 1986;44:805–815. doi: 10.1093/ajcn/44.6.805. [DOI] [PubMed] [Google Scholar]
- 46.Jaschonek K, Funck M, Muller CP, Schmidt H. Modulation of platelet [3H] iloprost binding by cholesterol, dibucaine and pentoxifylline. Prog Clin Biol Res. 1989;301:321–325. [PubMed] [Google Scholar]
- 47.Chabanel A, Flamm M, Sung KL, Lee MM, Schachter D, Chien S. Influence of cholesterol content on red cell membrane viscoelasticity and fluidity. Biophys J. 1983;44:171–176. doi: 10.1016/S0006-3495(83)84288-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, Simari R, Parton RG, Pagano RE. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell. 2004;15:3114–3122. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 50.Leslie M. Mysteries of the cell. Do lipid rafts exist? Science. 2011;334:1046– 1047. doi: 10.1126/science.334.6059.1046-b. [DOI] [PubMed] [Google Scholar]
- 51.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–698. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fallahi-Sichani M, Linderman JJ. Lipid raft-mediated regulation of G-protein coupled receptor signaling by ligands which influence receptor dimerization: a computational study. PLoS One. 2009;4:e6604. doi: 10.1371/journal.pone.0006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayoub MA, Pfleger KD. Recent advances in bioluminescence resonance energy transfer technologies to study GPCR heteromerization. Curr Opin Pharmacol. 2010;10:44–52. doi: 10.1016/j.coph.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Oh H, Mohler ER, 3rd, Tian A, Baumgart T, Diamond SL. Membrane cholesterol is a biomechanical regulator of neutrophil adhesion. Arterioscler Thromb Vasc Biol. 2009;29:1290–1297. doi: 10.1161/ATVBAHA.109.189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 56.Marquer C, Devauges V, Cossec JC, Liot G, Lecart S, Saudou F, Duyckaerts C, Leveque-Fort S, Potier MC. Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J. 2011;25:1295–1305. doi: 10.1096/fj.10-168633. [DOI] [PubMed] [Google Scholar]
- 57.Dalrymple MB, Pfleger KD, Eidne KA. G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol Ther. 2008;118:359–371. doi: 10.1016/j.pharmthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teitler M, Klein MT. A new approach for studying GPCR dimers: Drug-induced inactivation and reactivation to reveal GPCR dimer function in vitro, in primary culture, and in vivo. Pharmacol Ther. 2012;133:205–217. doi: 10.1016/j.pharmthera.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 61.Chakrabarti S, Liu NJ, Gintzler AR. Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proc Natl Acad Sci U S A. 2010;107:20115–20119. doi: 10.1073/pnas.1009923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong AH, Liu F. Uncoupling the Dopamine D1–D2 Receptor Complex: A Novel Target for Antidepressant Treatment. Clin Pharmacol Ther. 2012;91:298–302. doi: 10.1038/clpt.2011.311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


