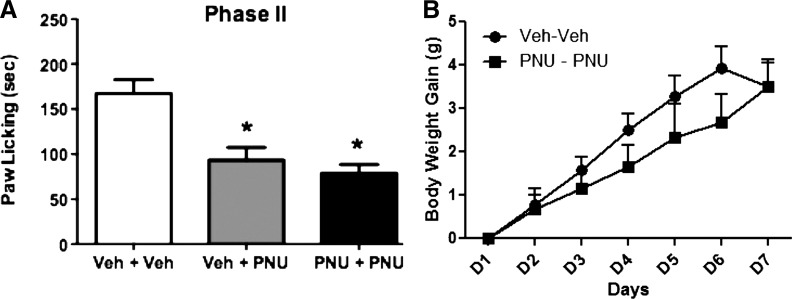
Fig. 9.
Lack of tolerance to PNU-120596–induced antinociception in the formalin test after subchronic administration of the drug. (A) Mice were treated with either vehicle or PNU-120596 (4 mg/kg i.p.) once a day for 6 days. On day 7, mice were challenged with PNU (4 mg/kg i.p.) and tested 15 minutes later during phase 2 of the formalin test. Control group received vehicle for 6 days, were challenged on day 7 with vehicle, and were tested 15 minutes later in the formalin test. (B) Lack of significant effect on the body weight change of mice after subchronic injection of PNU-120596. Body weight change (body weight on injection day − initial body weight before treatment) in the two treatment groups was recorded daily at the same time. Each bar represents the mean ± S.E.M. for each group of six to eight mice. *P < 0.05 versus vehicle-vehicle group. PNU, PNU-120596; Veh, vehicle.