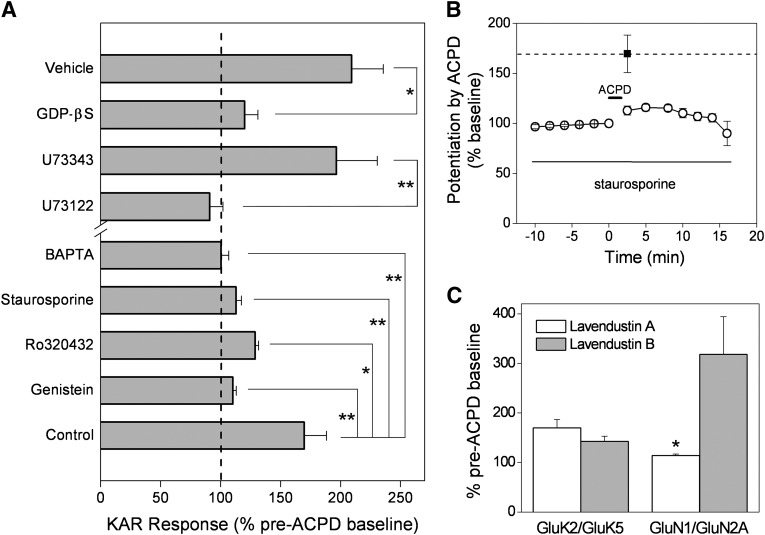
Fig. 6.
Dependence on G-protein, PLC, Ca2+, and PKC. (A) whole-cell currents were recorded from oocytes 3 days after injection of GluK2/GluK5/mGlu1 mRNAs. Before recording, injection of oocytes with 50 nl of GDPβS (10 mM) strongly reduced the ACPD-induced potentiation, compared with vehicle-injected cells (n = 5 and n = 12, respectively) (*P < 0.05; t test). The PLC inhibitor U73122 (10 µM) abolished ACPD-induced potentiation, but the inactive isomer U73343 (10 µM) did not (n = 10 and n = 8, respectively) (**P < 0.01; t test). Injection of BAPTA (100 mM, 100 nl) also blocked ACPD-induced potentiation of GluK2/GluK5/mGlu1 (n = 7). The broad spectrum kinase inhibitor staurosporine (100 nM), genistein (50 µM), and the specific PKC inhibitor Ro 320432 (2 µM) dramatically reduced ACPD-mediated potentiation in oocytes injected with GluK2/GluK5/mGlu1 (n ≥ 4 *P < 0.05, **P < 0.01; one-way ANOVA with Dunnett’s post-test, each compared with control). (B) the time-dependent potentiation of GluK2/GluK5/mGlu1 currents by ACPD was reduced by staurosporine (n ≥ 4). Note that exposure to staurosporine blocked the ACPD mediated potentiation during the entire recording. The filled square and dashed line indicates the normal peak level of potentiation (± S.E.M.) in the absence of staurosporine (n = 6). (C) pretreatment of oocytes with the specific src-tyrosine kinase inhibitor lavendustin A blocked ACPD-mediated potentiation of NMDA receptors (GluN1/GluN2A/mGlu1) (n = 3, *P < 0.05; t test) but failed to significantly inhibit the potentiation of GluK2/GluK5/mGlu1 (n = 9).