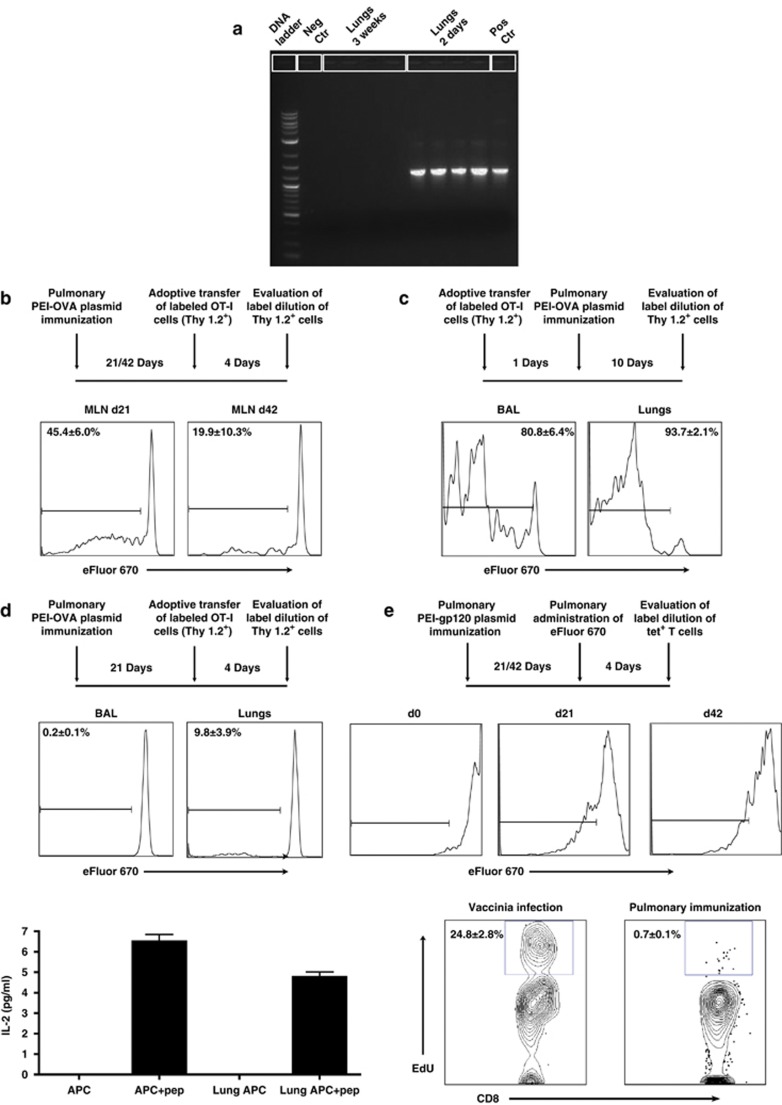
Figure 2.
Persistence of pulmonary CD8+ T cells is independent of the duration of antigen presentation in the airways and lungs or local T-cell proliferation. (a) Lungs were harvested from B6 mice 2 days and 3 weeks post pulmonary PEI-DNA-OVA immunization and plasmid DNA isolated from the tissue. The OVA sequence was amplified from plasmid DNA by PCR. The plasmid DNA-OVA used for immunization served as positive control (pos ctr) while plasmid DNA without the OVA insert served as the negative control (neg ctr). (b) B6.PL (Thy 1.1+) mice were immunized by the pulmonary route with PEI-DNA-OVA and 21 and 42 days later, received adoptively transferred labeled OT-I cells (Thy 1.2+) by intravenous injection. The dilution of eFluor 670 in donor Thy1.2+ tetramer+ CD8+ T cells was evaluated in mediastinal lymph nodes (MLN) of the immunized mice 4 days post transfer. Representative histograms show the average percentages±s.e. of proliferating Thy1.2+ tetramer+ CD8+ T cells (3–4 mice per group). (c) Labeled OT-I cells (Thy 1.2+) were adoptively transferred to the airway lumen of B6.PL mice (Thy 1.1+). One day later, mice were immunized by the pulmonary route with PEI-DNA-OVA, and 10 days later, donor tetramer-binding CD8+ T cells were isolated from the BAL and lungs, and evaluated for the dilution of eFluor 670 staining. (d) B6-PL (Thy 1.1+) mice were immunized by the pulmonary route with PEI-DNA-OVA and 21 days later received adoptively transferred labeled OT-I cells (Thy 1.2+) in their airway lumens. The dilution of eFluor 670 in donor Thy1.2+ tetramer+ CD8+ T cells was evaluated 4 days later in BAL and lungs. Representative histograms show the average percentages±s.e. of proliferating Thy1.2+ tetramer+ CD8+ T cells (6–8 mice per group). Local antigen presentation was also evaluated in lungs of B6 mice immunized by the pulmonary route with PEI-DNA-OVA. Lungs were harvested 6 weeks following immunization and co-cultured with the RF.33.70 hybridoma overnight. The levels of IL-2 secretion from the RF33.70 hybridoma in response to SIINFEKL presentation were determined using a mouse IL-2 enzyme-linked immunosorbent assay. Bars represent the average±s.e. of secreted IL-2 (3 mice per group). (e) Mice were inoculated by the pulmonary route with PEI-DNA-gp120, and 3 and 6 weeks later eFluor 670 was applied to the airways to stain local resident T cells. Four days later, the dilution of eFluor 670 staining of tetramer+ CD8+ T cells in the BAL was assessed. Histograms representative of 3–8 mice per time point are shown. Proliferation of antigen-specific CD8+ T cells was also evaluated in the BAL of immunized Balb/c mice 6 weeks following pulmonary PEI-DNA-gp120 immunization. Mice were injected with 250 μg 5-ethynyl-2′-deoxyuridine (EdU) intraperitoneally 12 h before sacrifice. Cells isolated from the BAL were stained with monoclonal antibodies to CD4 and CD8, fixed, permeabilized, and EdU detected with the Alexa Fluor 647 Click-iT EdU flow cytometry assay kit. Representative plot show the average percentages±s.e. of EdU incorporation by BAL CD8+ T cells (3 mice per group). BAL CD8+ T cells from mice infected intranasally with rVac-gp160 were used as a positive control for local proliferation. APC, antigen-presenting cells; BAL, broncho-alveolar lavage; ctr, control; IL-2, interleukin 2; pep, peptide; PEI-DNA-OVA, polyethyleneimine-DNA-Ovalbumin complexes.