Abstract
Patients with primary gastric lymphoma (PGL) are often treated with three-dimensional conformal radiotherapy (3D-CRT) in three to four fields to reduce the dose to the left kidney. However, the liver dose is higher than conventional parallel-opposed fields. This study was designed to evaluate hepatic dysfunction after 3D-CRT in patients with PGL. The data of 20 PGL patients treated with 3D-CRT were analyzed. Of the 20 patients, 3 had mucosa-associated lymphoid tissue (MALT) lymphoma and 17 had diffuse large B-cell lymphoma (DLBCL). The median dose used to treat MALT lymphoma was 30 Gy and 40 Gy for DLBCL. Pretreatment and post-treatment transaminase and alkaline phosphatase (ALP) values were compared. Radiation-induced hepatic dysfunction (RIHD) was defined as a more than 2-fold increase in transaminase or ALP levels, exceeding the upper limit within 4 months of the completion of radiotherapy. Increased transaminase or ALP levels were observed in 19 patients (95%). RIHD was observed in 14 patients (70%). The transaminase and ALP values were significantly different between pretreatment and post-treatment. There were significant differences in liver volumes receiving ≥5, ≥10, ≥15 and ≥20 Gy (V5, V10, V15 and V20) and in the mean liver doses between patients with and without RIHD. For patients with V10 > 60%, V15 > 50% or V20 > 30% in particular, the incidence rates of RIHD were significantly high. After radiotherapy for PGL, hepatic dysfunction occurred at a high rate. Thus, radiotherapy treatment should be planned in order to reduce liver doses.
Keywords: primary gastric lymphoma, radiotherapy, hepatic dysfunction
INTRODUCTION
In the past, primary gastric lymphoma (PGL) has been treated with surgery [1, 2]. Recently, radiotherapy with or without chemotherapy has been recommended as an alternative treatment method to radical gastrectomy in an attempt to preserve the stomach. Most patients with gastric lymphoma have mucosa-associated lymphoid tissue (MALT) lymphoma or diffuse large B-cell lymphoma (DLBCL) [3]. In gastric MALT lymphoma associated with Helicobacter pylori, eradicative antibiotic therapy results in a 55% to 80% complete regression [4–6]. Patients without evidence of H. pylori infection or with persistent lymphoma after eradicative antibiotic therapy are candidates for radiotherapy. Although the optimal treatment of localized gastric DLBCL remains controversial, stomach-preserving treatment with chemotherapy followed by radiotherapy is often applied.
Currently, three-dimensional conformal radiotherapy (3D-CRT) is a common method used in radiotherapy treatment. Many patients with PGL are treated in three to four fields in order to reduce the dose to the left kidney. This results in an increase of the liver dose compared to conventional anterior-posterior/posterior-anterior (AP/PA) parallel-opposed fields [7].
Radiation-induced liver disease (RILD) has been reported to be one of the most important complications in patients who undergo abdominal radiotherapy. RILD occurs within 4 months after the completion of irradiation and manifests as either anicteric elevation of alkaline phosphatase (ALP) level of at least 2-fold and non-malignant ascites (classic RILD), or elevated transaminases of at least 5-fold the upper limit of normal or of pretreatment level (non-classic RILD), in the absence of documented progressive disease [8–11]. In radiotherapy treatment for PGL, it has been speculated that the incidence of RILD is not high because the dose to the liver is low. However, irradiated patients with PGL often develop hepatic dysfunction. Many authors have described dose restrictions for liver [9–12]. These results were obtained from chronic hepatitis patient data. Dose restrictions to healthy liver are not obvious.
The purpose of this study was to analyze hepatic dysfunction after radiotherapy in patients with PGL and to determine the relationship of hepatic dysfunction to liver dose.
MATERIALS AND METHODS
Patient characteristics
Between August 2004 and May 2011, 30 patients with PGL received radiotherapy at our institution. Nine were excluded from this study because of the short follow-up period (<4 months). In addition, 1 was excluded because his pretreatment transaminase levels were slightly high. The remaining 20 patients were enrolled in this retrospective study. None of the patients had hepatic disorders, such as chronic hepatitis; 10 patients were male, and 10 patients were female. The median age was 65 ranging from 43 to 81. Of the 20 patients, 3 (15%) had MALT lymphoma, and 17 (85%) had DLBCL. Two of the 3 patients with MALT lymphoma had the chromosomal translocation t(11;18)(q21;q21) and were resistant to antibiotic therapy. H. pylori was negative for the remaining patient.
Treatment plan
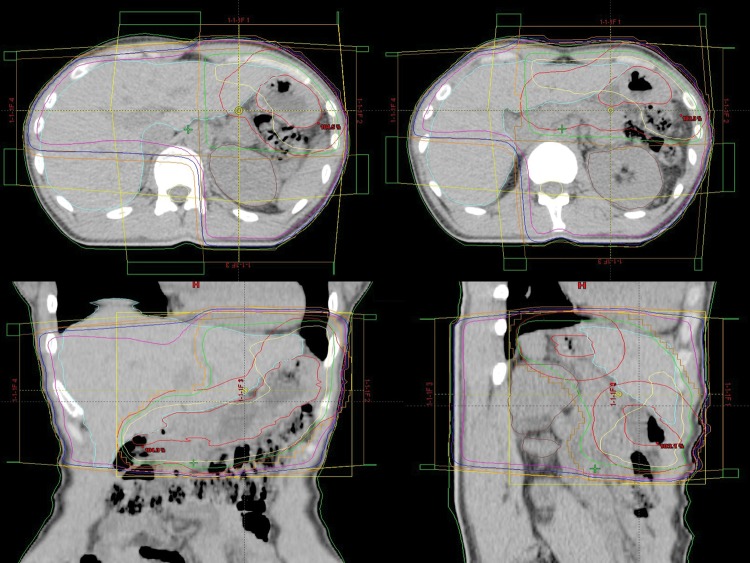
The median dose given to the patients with MALT lymphoma was 30 Gy (range, 30–36 Gy). All patients with DLBCL received three to six cycles of chemotherapy followed by radiotherapy. The chemotherapy consisted of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. The median dose given to the patients with DLBCL was 40 Gy (range, 36–41.4 Gy). The fraction size ranged from 1.8 to 2 Gy (median, 2 Gy). The clinical target volume (CTV) was the entire stomach. The planning target volume was defined as the CTV plus an approximate 2-cm margin in all directions. A total of 18 patients (90%) were treated with four fields and 2 patients (10%) were treated with 3 fields. A representative example of the treatment plan is presented (Fig. 1).
Fig. 1.
A representative example of a four fields treatment plan. Isodose lines depicted are 100% (yellow), 95% (green), 50% (purple), 37.5% (blue) and 25% (orange).
Evaluation of hepatic dysfunction
None of the patients exhibited increased levels of transaminase or ALP in the pretreatment blood test. The pretreatment blood test was examined 0–10 days before the start of radiotherapy (median, 1 day). Patients had blood tests 2 weeks, 1 month, 2 months, 3 months and 4 months after radiotherapy in order to evaluate transaminase and ALP levels. Cases where transaminase or ALP levels exceed the normal upper limits slightly after irradiation are not of clinical significance. Cases where transaminase or ALP levels elevate more than double pretreatment values and exceed normal limits after irradiation are also not of clinical significance if the pretreatment values were low. Therefore, radiation-induced hepatic dysfunction (RIHD) was defined as a more than 2-fold increase of transaminase or ALP levels exceeding the upper limits within 4 months after the completion of radiotherapy.
A dose-volume histogram (DVH) was generated from every treatment plan. The percentages of liver volumes receiving at least 5 Gy, 10 Gy, 15 Gy, 20 Gy, 25 Gy and 30 Gy (V5, V10, V15, V20, V25 and V25, respectively) and the mean dose to the liver were calculated.
Wilcoxon signed-rank tests were used to compare the transaminase and ALP values between pretreatment and post-treatment. Patients were classified into two groups according to the presence of RIHD. The liver doses of these two groups were assessed with Mann-Whitney U test. Bivariate analyses of the association between liver dose and RIHD were performed with Fisher's exact probability tests. Ages of patients with RIHD and without RIHD were compared using Mann-Whitney U test. Associations between RIHD and sex or the presence of chemotherapy were analyzed with Fisher's exact probability tests. A P value <0.05 was considered to indicate a significant difference.
RESULTS
The median follow-up time was 32 months (range, 6–79 months). No relapses were observed during the follow-up period.
Radiation-induced hepatic dysfunction
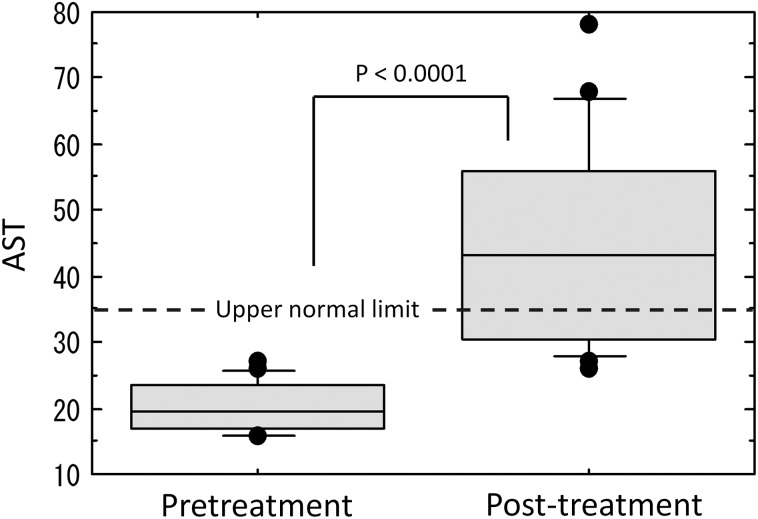
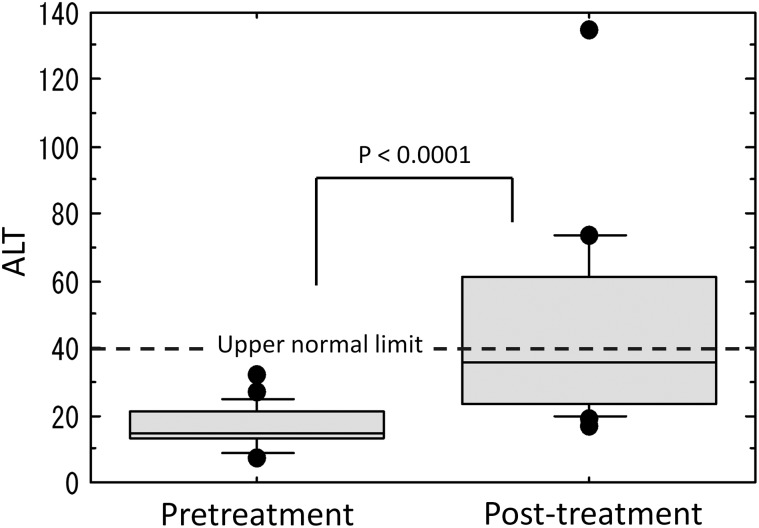
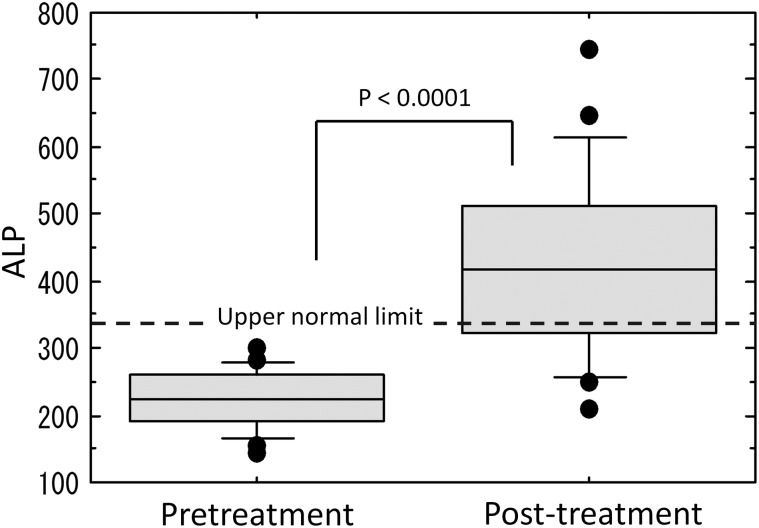
Increased levels of transaminase were observed in 14 patients (70%), increased levels of ALP were observed in 14 patients (70%), and increased levels of transaminase or ALP were observed in 19 patients (95%). Maximum values of transaminase and ALP were observed, on average, 1.9 months and 2.4 months, respectively, after radiotherapy completion. The transaminase and ALP values between pretreatment and post-treatment were significantly different (Figs. 2–4).
Fig. 2.
Comparison of aspartate aminotransferase (AST) levels between pretreatment and post-treatment.
Fig. 3.
Comparison of alanine aminotransferase (ALT) levels between pretreatment and post-treatment.
Fig. 4.
Comparison of alkaline phosphatase (ALP) levels between pretreatment and post-treatment.
A more than 2-fold increase in the levels of transaminase was observed in 14 patients (70%); ALP in 8 patients (40%); and transaminase or ALP in 16 patients (80%). A more than 2-fold increase and exceeding the upper normal limits in transaminase was observed in 12 patients (60%), in ALP was observed in 7 patients (35%), and in transaminase or ALP, indicating RIHD, was observed in 14 patients (70%).
All of the 14 patients with transaminase abnormalities had improved by 2–50 months (median, 5.5 months). Of the 14 patients with ALP abnormalities, 8 patients (57%) had improved by 3–21 months (median, 10.5 months). In the remaining 6 patients (43%), abnormal ALP levels continued within the follow-up period.
There was no statistically significant difference between the ages of patients with RIHD and without RIHD. There was no statistically significant relationship between RIHD and sex or the presence of chemotherapy.
Liver dose and hepatic dysfunction
There were significant differences in V5, V10, V15, V20 and the mean dose to liver between patients with RIHD and patients without RIHD (Table 1). For patients with V10 > 60%, V15 > 50%, or V20 > 30% in particular, the incidence rates of RIHD were significantly increased (Fisher's exact probability test, all P values < 0.05) (Table 2–4). There were no significance differences in liver dose between patients in whom the increased ALP levels had not improved and other patients.
Table 1.
Dosimetric parameters for patients with RIHD and without RIHD
| with RIHD (n = 14) | without RIHD (n = 6) | P value | |
|---|---|---|---|
| V5 | 83.0 ± 7.5 | 69.5 ± 12.6 | 0.0209 |
| V10 | 71.0 ± 9.1 | 44.4 ± 24.2 | 0.0149 |
| V15 | 60.8 ± 12.5 | 38.8 ± 22.1 | 0.0209 |
| V20 | 44.3 ± 18.9 | 24.7 ± 11.9 | 0.0478 |
| V25 | 18.9 ± 4.9 | 19.1 ± 7.5 | 0.6801 |
| V30 | 10.9 ± 5.3 | 13.6 ± 8.1 | 0.3223 |
| Mean dose | 17.3 ± 2.4 | 14.6 ± 2.5 | 0.0319 |
RIHD = radiation-induced hepatic dysfunction; V5, V10, V15, V20, V25 and V30 = percentage of liver volumes receiving ≥ 5, ≥ 10, ≥ 15, ≥ 20, ≥ 25 and ≥ 30 Gy, respectively
Table 2.
Two-by-two frequency table of V10 for patients with and without RIHD
| with RIHD (n = 14) | without RIHD (n = 6) | |
|---|---|---|
| V10 > 60% | 12 | 2 |
| V10 ≤ 60% | 2 | 4 |
RIHD = radiation-induced hepatic dysfunction, V10 = percentage of liver volumes receiving ≥ 10 Gy
Table 4.
Two-by-two frequency table of V20 for patients with and without RIHD
| with RIHD (n = 14) | without RIHD (n = 6) | |
|---|---|---|
| V20 > 30% | 10 | 1 |
| V20 ≤ 30% | 4 | 5 |
RIHD = radiation-induced hepatic dysfunction, V20 = percentage of liver volumes receiving ≥ 20 Gy
Table 3.
Two-by-two frequency table of V15 for patients with and without RIHD
| with RIHD (n = 14) | without RIHD (n = 6) | |
|---|---|---|
| V15 > 50% | 12 | 2 |
| V15 ≤ 50% | 2 | 4 |
RIHD = radiation-induced hepatic dysfunction, V15 = percentage of liver volumes receiving ≥ 15 Gy
DISCUSSION
In the past, PGL has been treated with surgery, consisting of partial or total gastrectomy [1, 2]. Recently, radiotherapy with or without chemotherapy has been recommended as an alternative treatment method to radical gastrectomy in an attempt to preserve the stomach.
DLBCL and MALT lymphoma account for about 60% and 38%, respectively, of PGL [3]. Other histologic subtypes are rare. About 70% to 90% of patients with MALT lymphoma present with localized disease [13, 14]. Gastric MALT lymphoma develops in response to stimuli such as the presence of H. pylori. In gastric MALT lymphoma associated with H. pylori, eradicative antibiotic therapy results in 55–80% complete regression [4–6]. Tumors with the chromosomal translocation t(11;18)(q21;q21) in patients with gastric MALT lymphoma are usually resistant to antibiotic therapy [15, 16]. Radiotherapy is applied to patients with persistent or progressive gastric MALT lymphoma after H. pylori eradication. Radiotherapy for localized gastric MALT lymphoma resulted in a 96– 100% complete regression (CR) [17, 18]. Although the optimal treatment of localized gastric DLBCL still remains controversial, stomach-preserving treatment with chemotherapy followed by radiotherapy is often applied. Ishikura et al. reported that the complete response rate, the 2-year progression-free survival rate, and the 2-year overall survival (OS) rate were 92%, 88% and 94%, respectively [19]. Koch et al. reported that the event-free survival and OS rates at 42 months were 84.5% and 87.0%, respectively [3].
Renal toxicity is an important complication of radiotherapy for the treatment of PGL [20]. Radiotherapy with AP/PA parallel-opposed fields raises the risk of radiation nephropathy and hypertension. Currently, 3D-conformal radiotherapy (3D-CRT) is the most common method used in radiotherapy treatment. Patients with PGL are often treated with a 3- to 4-field treatment plan in order to reduce the dose to the left kidney. This results in an increase in the liver dose compared to that in AP/PA parallel-opposed fields. Biancia et al. reported that the mean liver dose for 4-field 3D-CRT was 17.6 Gy compared to 6.8 Gy in AP/PA parallel-opposed fields [7].
RILD has been reported to be one of the most important complications for patients who undergo abdominal radiotherapy. The pathologic changes in liver after radiotherapy have been estimated as follows [21]. First, fibrin deposits in the central veins and in the afferent sinusoids. Transforming growth factor β stimulates fibroblasts that migrate to this site, proliferate and produce collagen. Then deposition of collagen causes congestion of the sinusoids in the central portion of the lobules with atrophy of the inner portion of the liver plates. In most cases the liver gradually heals and shows little congestion. However, distortion of the lobular architecture often remains. The degree of healing and the presence of RILD varies depending on the case.
Many authors have reported dose restrictions for the liver. Cheng et al. reported that the frequency of RILD among patients who received a mean dose greater than 28 Gy to the liver was greater than that among patients who received a mean dose less than 28 Gy [9]. Liang et al. reported that a mean dose to the liver of 23 Gy was the tolerance dose for Child-Pugh Grade A patients [10]. Those authors also reported that a V20 of 48.5% was a liver tolerance dose [11]. Kim et al. reported that a V30 of 60% was a significant parameter associated with a risk of Common Terminology Criteria for Adverse Events, Grade 2, hepatic toxicity [12]. These results were obtained from data from patients with chronic hepatitis. Dose restrictions for healthy liver are not known.
None of the patients enrolled in the present study had hepatic diseases such as chronic hepatitis or hepatic dysfunction, according to their pretreatment blood tests. The prescription dose was relatively low at about 30 to 40 Gy, and radiotherapy did not target the liver itself. However, the maximum transaminase and ALP levels exceeded the upper normal limit with the high rate of 95%, and 70% of the patients developed RIHD. Furthermore, 1 developed non-classic RILD. There were significant differences in V5, V10, V15 and V20 between patients with RIHD and those without RIHD. Especially for patients with V10 > 60%, V15 > 50% or V20 > 30%, the incidence rates of RIHD were significantly higher. Further study is necessary in order to discuss dose restrictions for liver because of the small number of cases in the current study. However, kidney doses and liver doses should be as low as possible.
Of the 14 patients with ALP levels that were over the upper normal limits, abnormal ALP levels continued within the follow-up period in 43% of the patients. If a patient has other disease unrelated to PGL after radiotherapy, hepatic dysfunction and its prolongation might disturb their treatment. This risk seems to be relatively high because of the good prognosis associated with PGL.
Watchful observation is needed after radiotherapy treatment for PGL, keeping in mind that hepatic dysfunction occurs at a high rate after irradiation. Radiotherapy treatment should be planned in order to reduce liver doses, and dose restrictions for healthy liver should be further evaluated.
REFERENCES
- 1.Durr ED, Bonner JA, Strickler JG, et al. Management of stage IE primary gastric lymphoma. Acta Haematol. 1995;94:59–68. doi: 10.1159/000203975. [DOI] [PubMed] [Google Scholar]
- 2.Roukos DH, Hottenrott C, Encke A, et al. Primary gastric lymphomas: a clinicopathologic study with literature review. Surg Oncol. 1994;3:115–25. doi: 10.1016/0960-7404(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 3.Koch P, Probst A, Berdel WE, et al. Treatment results in localized primary gastric lymphoma: Data of patients registered with the German multicenter study (GIT NHL 02/96) J Clin Oncol. 2005;23:7050–9. doi: 10.1200/JCO.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Bayerdorffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue subtype after cure of Helicobacter pylori infection: MALT Lymphoma Study Group. Lancet. 1995;345:1591–4. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 5.Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and elevation of the diagnostic protocol's adequacy. Recent Results Cancer Res. 2000;156:116–24. doi: 10.1007/978-3-642-57054-4_15. [DOI] [PubMed] [Google Scholar]
- 6.Bertoni F, Conconi A, Capella C, et al. Molecular follow-up in gastric mucosa-associated lymphoid tissue lymphomas: early analysis of the LY03 cooperative trial. Blood. 2002;99:2541–4. doi: 10.1182/blood.v99.7.2541. [DOI] [PubMed] [Google Scholar]
- 7.Della Biancia C, Hunt M, Furhang E, et al. Radiation treatment planning techniques for lymphoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;62:745–51. doi: 10.1016/j.ijrobp.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–8. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J-C, Wu J-K, Huang C-M, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156–62. doi: 10.1016/s0360-3016(02)02915-2. [DOI] [PubMed] [Google Scholar]
- 10.Liang S-X, Zhu X-D, Xu Z-Y, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426–34. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Liang S-X, Huang X-B, Zhu X-D, et al. Dosimetric predictor identification for radiation-induced liver disease after hypofractionated conformal radiotherapy for primary liver carcinoma patients with Child-Pugh Grade A cirrhosis. Radiother Oncol. 2011;98:265–9. doi: 10.1016/j.radonc.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Kim T-H, Kim D-Y, Park J-W, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:225–31. doi: 10.1016/j.ijrobp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Thieblemont C, Bastion Y, Berger F, et al. Mucosa-associated lymphoid tissue gastrointestinal, and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997;15:1624–30. doi: 10.1200/JCO.1997.15.4.1624. [DOI] [PubMed] [Google Scholar]
- 14.Zucca E, Roggero E, Pileri S. B-cell lymphoma of MALT type: a review with special emphasis on diagnostic and management problems of low-grade gastric tumors. Br J Haematol. 1998;100:3–14. doi: 10.1046/j.1365-2141.1998.00513.x. [DOI] [PubMed] [Google Scholar]
- 15.Ye H, Liu H, Raderer M, et al. High incidence of t(11;18)(q21;q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood. 2003;101:2547–50. doi: 10.1182/blood-2002-10-3167. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. 2001;357:39–40. doi: 10.1016/S0140-6736(00)03571-6. [DOI] [PubMed] [Google Scholar]
- 17.Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998;16:1916–21. doi: 10.1200/JCO.1998.16.5.1916. [DOI] [PubMed] [Google Scholar]
- 18.Tsang R-W, Gospodarowicz MK, Pintilie M, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21:4157–64. doi: 10.1200/JCO.2003.06.085. [DOI] [PubMed] [Google Scholar]
- 19.Ishikura S, Tobinai K, Ohtsu A, et al. Japanese multicenter phase II study of CHOP followed by radiotherapy in stage I-II, diffuse large B-cell lymphoma of the stomach. Cancer Sci. 2005;96:349–52. doi: 10.1111/j.1349-7006.2005.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luxton RW. Radiation nephritis. A long-term study of 54 patients. Lancet. 1961;2:1221–1224. doi: 10.1016/s0140-6736(61)92590-9. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–48. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]