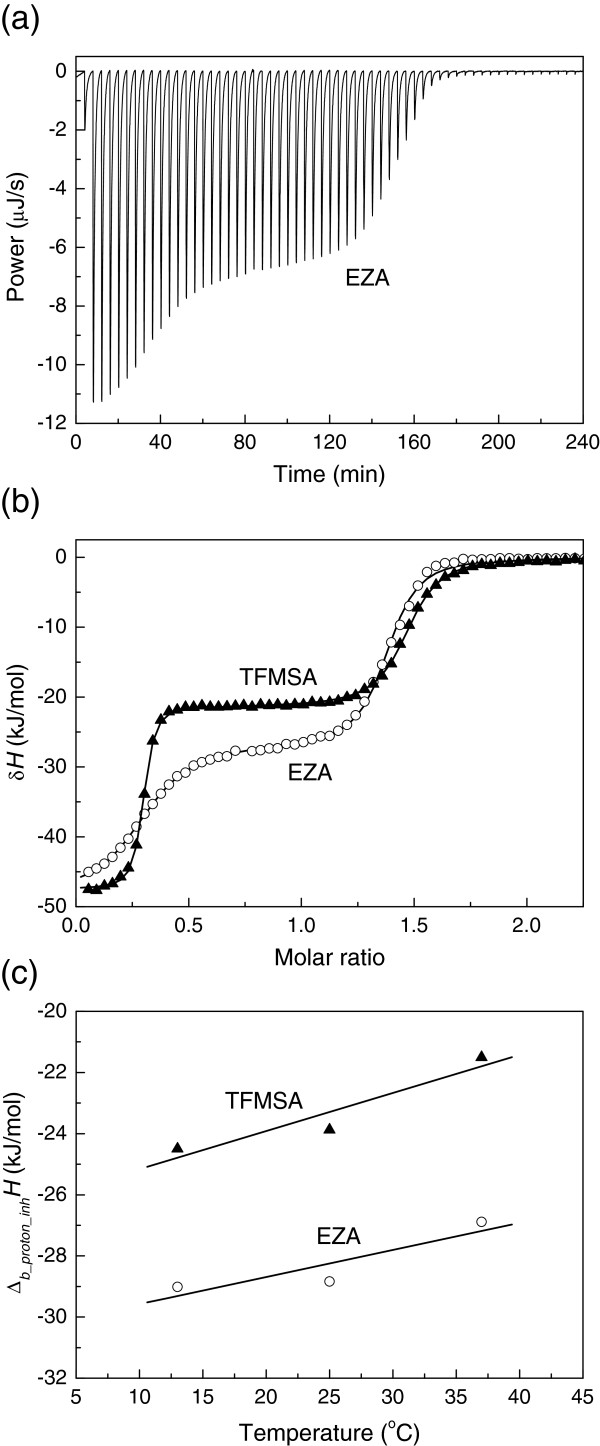
Figure 6.
Determination of the EZA protonation enthalpy. (a) ITC raw titration curve of 0.25 mM EZA in water with 1.5 equivalent NaOH titrated with 2.5 mM HNO3 at 37°C. (b) Integrated ITC curves of EZA (◯) and TFMSA (▲) titration with HNO3 at 37°C. The first transition represents the neutralization of 0.5 equivalent of NaOH with HNO3 while the second transition (between the molar ratio of 0.5 and 1.5) represents the titration of the compound. In these experiments the enthalpies of protonation were −21.3 kJ/mol for TFMSA and −27.6 kcal/mol for EZA. (c) The enthalpies of EZA (◯) and TFMSA (▲) protonation as a function of temperature. The slopes yield the heat capacities of protonation equal to 88.7 J/(mol × K) for EZA and 124.7 J/(mol × K) for TFMSA.