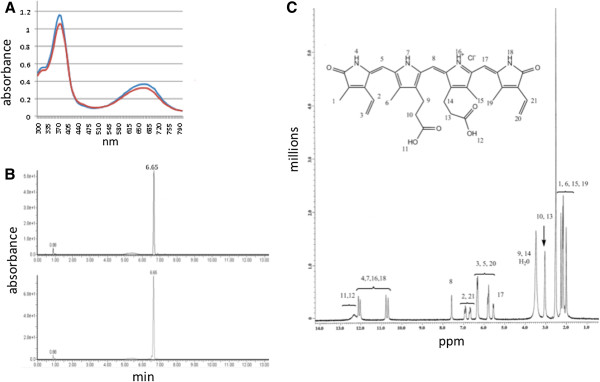
Figure 5.
Spectral and chromatographic analyses of biliverdin produced by bioreactor cultures ofE. coliBL21 (mHO1). (A) Absorbance spectra of biliverdin produced by E. coli BL21 (mHO1) (red) and commercial biliverdin IXα derived from an animal source (blue). (B) HPLC chromatograms of biliverdin produced by E. coli BL21 (mHO1) (top) and commercial biliverdin IXα derived from an animal source (bottom). (C) One dimensional proton NMR (400 Mhz) spectrum of E. coli BL21 (mHO1)-produced biliverdin in DMSO-d6. 1H NMR (400 MHz, DMSO) signal assignments are: 12.32 (s; 2H); 12.1(s; 1H); 12.01 (s; 1H); 10.78 (s; 1H); 10.67 (s; 1H); 7.58 (s; 1H); 6.91 (t, J= 15.6 Hz; 1H); 6.69 (t, J= 15.2 Hz; 1H); 6.32 (d, J= 12.2 Hz; 3H); 5.82 (d, J=10.8 Hz; 1H); 5.56 (d, J= 11.2 Hz; 1H); 5.78 (s; 1H); 3.05 (m; 4H); 3.43 (m; 4H); 2.18 (s; 3H); 2.27 (s; 3H); 2.15 (s; 3H); 2.01 (s; 3H). The spectrum is similar to biliverdin IXα derived from animal sources [36].