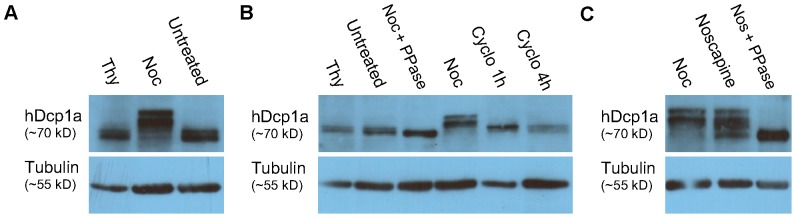
Figure 4. Dcp1a is hyper-phosphorylated during cell division.
Western blot analysis of (A) endogenous hDcp1a protein in U2OS cell extracts during interphase (untreated), metaphase (nocodazole block, Noc) and at G1/S (thymidine block, Thy), showed the appearance of slower migrating Dcp1a bands in metaphase cells. (B) Treatment of U2OS protein extracts from metaphase cells with a phosphatase (Noc+PPase) caused a reduction in the molecular weight of hDcp1a, compared to untreated, G1/S blocked (Thy), and metaphase blocked cells (Noc). This demonstrated that Dcp1a is hyper-phosphorylated during mitosis. Treatment with cycloheximide (Cyclo) for 1 or 4 hrs did not change the mobility of hDcp1a indicating that hyper-phosporylation is cell cycle dependent. (C) Shift in mobility due to hyper-phosphorylation in mitotic cells is seen using two different cell cycle blockers, nocodazole (Noc) and noscapine. Similarly, phosphatase treatment (Nos+PPase) caused a reduction in the molecular weight of Dcp1a from noscapine treated cells. Tubulin was used as a loading control.