Figure 8.
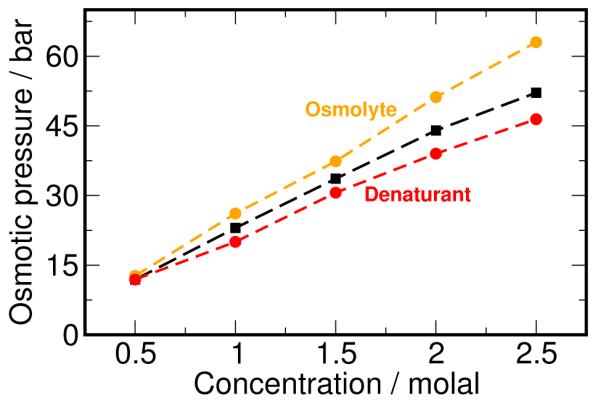
Osmotic behavior of cosolvents. We hypothesize that osmolytes (orange) show a positive deviation from the van’t Hoff law (black), while denaturants (red) show a negative deviation from the same.

Osmotic behavior of cosolvents. We hypothesize that osmolytes (orange) show a positive deviation from the van’t Hoff law (black), while denaturants (red) show a negative deviation from the same.