Figure 10. MAO-A regulates tyramine-induced ROS generation in IL-13 and IL-4-activated monocytes/macrophages.
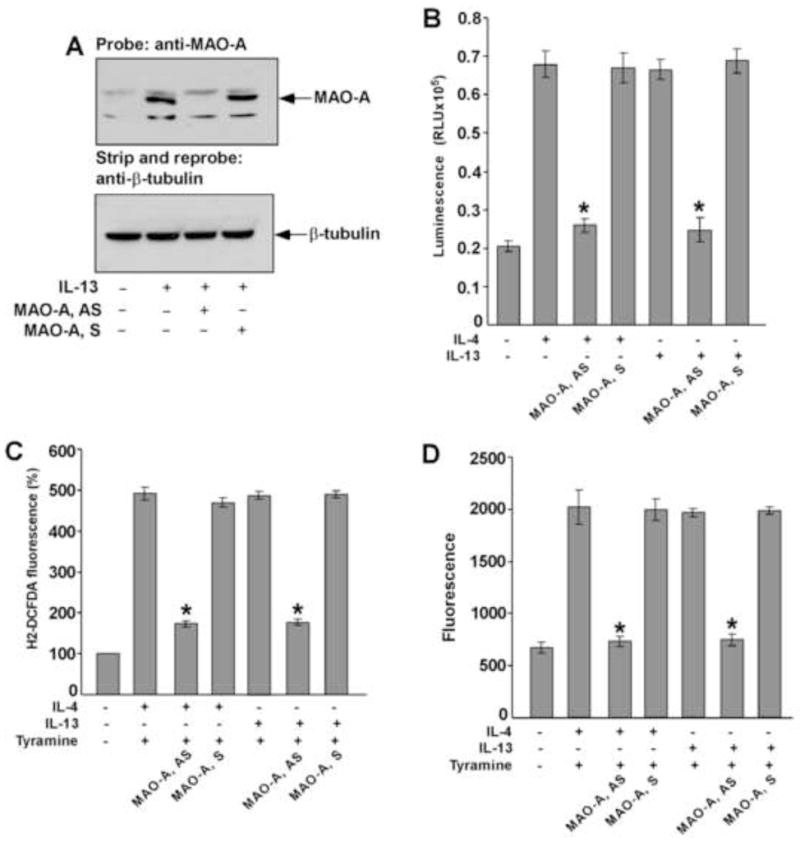
Monocytes (5×106/group) were pre-treated with MAO-A sense (S) or antisense (AS) ODN (A–D) according to protocols described under “Materials and Methods” and incubated with IL-13 (A–D) and IL-4 (B–D) (2nM) for 46h. In panel A, whole cell extracts (50 μg/lane) were resolved by SDS-PAGE. MAO-A protein expression was detected on Western blots with a MAO-A specific antibody (upper panel of A). The blot was stripped and reprobed with an antibody against (β-tubulin (lower panel of A) to assess equal loading. In panel B, native MAO-A enzyme activity was detected. In a total volume of 50 μl, 20 μl whole cell extract was incubated with substrate (final conc. 40 μM) in presence of MAO reaction buffer [100mM HEPES (pH 7.5); 5% glycerol]. After incubation at room temperature for 3h, 50μl luciferin detection reagent was added and the luminescent signal was measured after 20 min. Data represented as mean ± SD; (n=3, *p<0.002). In panel C, monocytes/macrophages were stimulated by tyramine (5 μM) for 30 min and then incubated with the fluorescent probe H2DCFDA (5 μM) for another 30 min before the fluorescence was measured. Data in panel C is expressed as percentage of unstimulated controls and represented as mean ± SD; (n=3, *p<0.004). In panel D, monocyte lysates (50μg/well) were incubated with Amplex Red reagent/HRP/p-Tyramine working solution for 60 min before the fluorescence was measured. Data represented as mean ± SD; (n=3, *p<0.005). Data in all panels are from a representative of three independent experiments.
