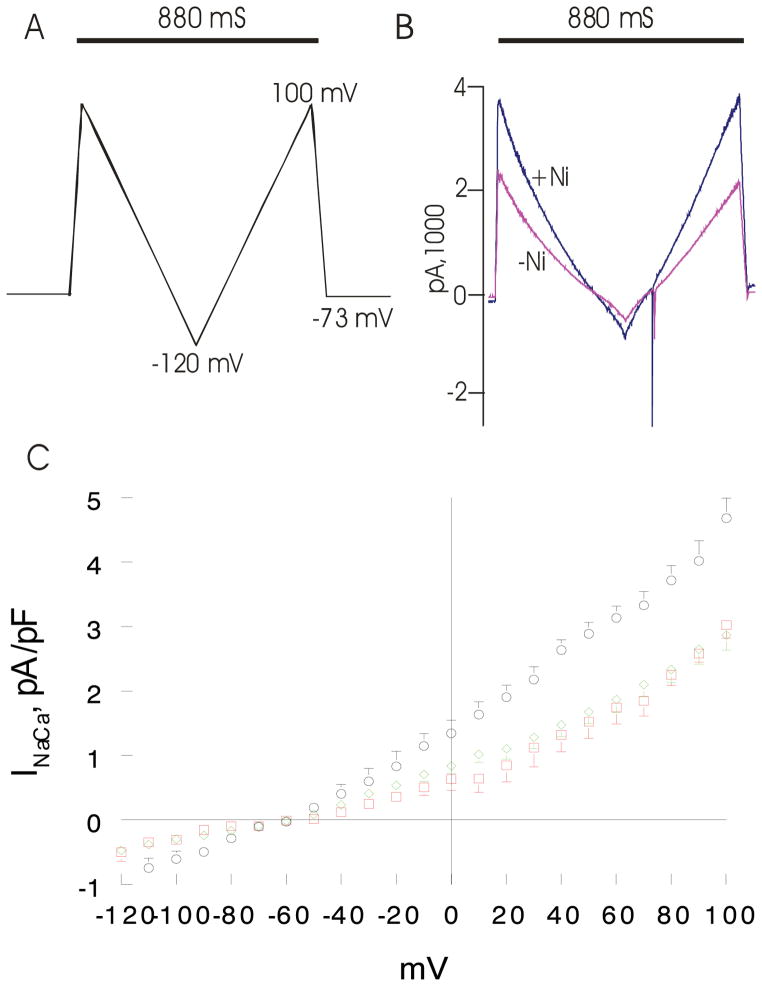
Figure 4. Effects of induced overexpression of Na+/Ca2+ exchanger (NCX1) and transverse aortic constriction (TAC) on NCX1 current (INaCa).
Pipette solution contained (in mM) 100 Cs+ glutamate, 7.25 NaCl, 1 MgCl2, 20 HEPES, 2.5 Na2ATP, 10 EGTA and 6 CaCl2, pH 7.2. Free Ca2+ in the pipette solution was 205 nM, measured fluorimetrically with fura-2. External solution contained (in mM) 130 NaCl, 5 CsCl, 1.2 MgSO4, 1.2 NaH2PO4, 5 CaCl2, 10 HEPES, 10 Na+ HEPES and 10 glucose, pH 7.4. Verapamil (1 μM) was used to block ICa. Our measurement conditions were biased towards measuring outward (3 Na+ out: 1 Ca2+ in) INaCa. (A). After holding the myocyte at the calculated reversal potential (−73 mV) of INaCa for 5 min. (to minimize fluxes through NCX1 and thus allowed [Na+]i and [Ca2+]i to equilibrate with those in pipette solution), INaCa (30°C) was measured in WT-sham, WT-TAC and NCX1-TAC myocytes using a descending (from +100 to −120 mV; 500 mV/s) - ascending (from −120 to +100 mV; 500 mV/s) voltage ramp, first in the absence and then in the presence of 1 mM NiCl2. (B). Raw currents measured in a WT-sham myocyte. INaCa was defined as the difference current measured in the absence and presence of Ni+ during the descending voltage ramp. Note that with the exception of small contamination of the ascending ramp by the cardiac Na+ current, there were little to no differences in currents measured between the descending and ascending voltage ramps. This suggests that [Ca2+]i and [Na+]i sensed by NCX1 did not appreciably change by NCX1 fluxes during the brief (880 ms) voltage ramp. INaCa was divided by Cm prior to comparisons. (C). Current-voltage relationships of INaCa (means ± SE) from WT-sham ( ;n=7 myocytes from 4 mice), WT-TAC (◇ n=10 myocytes from 3 mice) and NCX1-TAC (○; n=8 myocytes from 3 mice) myocytes are shown. The reversal potential of INaCa was ~−60 mV, close to the theoretical reversal potential of −73 mV. Error bars are not shown if they fall within the boundaries of the symbol.