Table 2.
Structures and activities of analogs 24a–k attached to the preferred biaryl.

| ||||
|---|---|---|---|---|
| Compd | hM1 EC50 (μM)a | AChmax (%)a,b | pEC50a | |
| 24a |

|
5.6 | 44 ± 3 | 5.25 ± 0.07 |
| 24b |
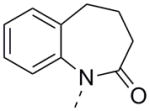
|
- | ||
| 24c |

|
>10 | 51 ± 3 | |
| 24d |

|
- | ||
| 24e |

|
5.2 | 52 ± 3 | 5.29 ± 0.09 |
| 24f |

|
>10 | 62 ± 2 | |
| 24g |
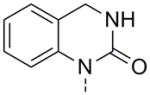
|
>10 | 53 ± 2 | |
| 24h |

|
3.2 | 30 ± 2 | 5.49 ± 0.12 |
| 24i |

|
>10 | 46 ±3 | |
| 24j |

|
>10 | 36± 2 | |
| 24k |

|
- | ||
Values represent the mean ± standard error of the means of at least three independent determinations performed in triplicate.
“-” indicates an inactive compound showing no PAM activity up to the highest concentration tested (30 μM).
When hM1 EC50 is reported as >10, this value represents the percent maximal ACh response when the test compound was present at its highest concentration (30 μM).
