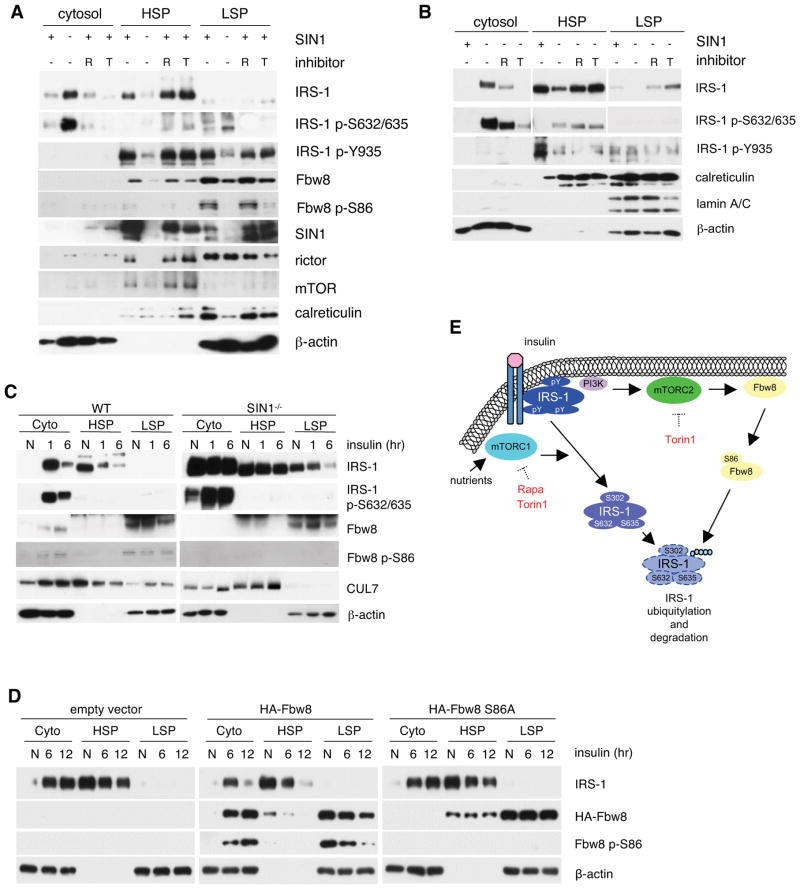
Figure 7.
Disruption of mTORC2 promotes accumulation of inactive IRS-1 in the cytosol and exclusion of Fbw8 from this compartment. A–B. Growing WT or SIN1−/− MEFs were treated with either 100 nM rapamycin (R) or 250 nM Torin1 (T). Cells were homogenized and extracts were fractionated into cytosol, high-speed (HSP) and low-speed pellet (LSP). Fractions were analyzed by Western blotting with 20 μg of protein loaded in each lane. C. WT or SIN1−/− were grown in normal media (N), starved and restimulated with insulin for either 1 or 6 hrs. Cells were processed as in A. D. Fbw8−/− MEFs were transfected with either empty vector or Fbw8 constructs as indicated. Cells were processed as in C above. E. Model for IRS-1 downregulation by mTORC1 vs mTORC2. Tyrosine-phosphorylated-IRS-1 on the membrane becomes Ser phosphorylated at several residues including the mTORC1/S6K-mediated sites after prolonged insulin stimulation. mTORC2 and Fbw8 colocalize at the membrane under basal conditions where mTORC2 phosphorylates Ser86 to stabilize Fbw8 and promotes its cytosolic localization upon insulin stimulation. During prolonged insulin stimulation, inactive serine-phosphorylated IRS-1 and Fbw8 colocalize to the cytosol where the former becomes ubiquitylated via Fbw8 and undergoes proteasomal degradation.