Abstract
Studies of depression and hepatitis C virus (HCV) infection in HIV-infected patients have been contradictory and often not addressed key differences between HCV-infected and uninfected individuals including substance use. This cross-sectional observational study from the University of Washington HIV Cohort examined associations between HCV, symptoms, and depression in HIV-infected patients in routine clinical care. Patients completed instruments measuring depression, symptoms, and substance use. We generated depression severity scores and used linear regression to examine the relationship with HCV accounting for demographic and clinical characteristics. We conducted sensitivity analyses in which we removed depression somatic items (e.g. fatigue) from depression scores, and sensitivity analyses in which we also adjusted for non-depression somatic symptom items to examine the role of somatic and non-somatic symptoms in the association between depression and HCV. Of 764 HIV-infected patients, 160 (21%) were HCV-infected. In adjusted analysis, HCV-infected patients had worse depression severity (p=0.01) even after adjusting for differences in substance use. HCV remained associated with depression severity in secondary analyses that omitted the depression somatic PHQ-9 items (p = 0.01). However, when non-depression somatic symptoms were included as covariates in multivariate analyses, HCV was no longer associated with depression (p = 0.09).
Conclusions
We found a high prevalence and severity of depression among HIV-infected patients in routine care, particularly among those with HCV. The association between HCV and depression persisted even when depression somatic PHQ-9 items were omitted suggesting the association was not due to misclassification of HCV-related somatic symptoms like fatigue as depression. However, in models that also adjusted for non-depression somatic symptoms, the association disappeared highlighting the strong relationship between symptom burden and depression. Longitudinal studies are needed to assess the degree symptoms mediate the association between HCV and depression, and whether increased symptom burden is due in part to depression.
Keywords: hepatitis C virus, depression, HIV, somatic symptoms, antidepressant medications
INTRODUCTION
Understanding the connection between hepatitis C virus (HCV) infection and depression in HIV-infected patients is critical given the effect of depression on quality of life, adherence to antiretroviral therapy, and the decision to initiate HCV treatment(Braitstein, et al., 2005; Buti, Wong, Casado, & Esteban, 2006; Fleming, et al., 2004; Kanwal, et al., 2005; Starace, et al., 2002). In the United States, HCV infection is more common among HIV-infected than uninfected individuals with a reported prevalence rate between 16–37%(Kim, Psevdos, Suh, & Sharp, 2008; Sherman, Rouster, Chung, & Rajicic, 2002; Staples, Rimland, & Dudas, 1999). Previous studies examining the occurrence of depressive symptoms in patients co-infected with HIV and HCV have produced conflicting results with some finding an association(Backus, Boothroyd, & Deyton, 2005; Baum, et al., 2008; Libman, et al., 2006; Mrus, et al., 2006), and others not(Baillargeon, et al., 2008; Grassi, et al., 2002; Richardson, et al., 2005; Ryan, Morgello, Isaacs, Naseer, & Gerits, 2004; Thein, et al., 2007; von Giesen, et al., 2004). These different results might be explained by confounding factors, the use of different depression scales, small samples, and variable prevalence of HCV treatment that can itself precipitate depression. Compared to HIV-infected patients without HCV, HIV and HCV co-infected individuals tend to be older(Backus, et al., 2005; Kanwal, et al., 2005; Mrus, et al., 2006; Staples, et al., 1999; Sulkowski, Moore, Mehta, Chaisson, & Thomas, 2002), and are more likely to be male(Backus, et al., 2005; Kanwal, et al., 2005; Kim, et al., 2008), African-American(Kanwal, et al., 2005; Kim, et al., 2008; Staples, et al., 1999; Sulkowski, et al., 2002) or Hispanic(Backus, et al., 2005), and to have a history of alcohol abuse or illicit drug use(Backus, et al., 2005; Kanwal, et al., 2005; Kim, et al., 2008; Staples, et al., 1999; Sulkowski, et al., 2002). Some of these factors, particularly substance use, are in turn associated with depression(Kessler, et al., 2003; L. E. Sullivan, et al., 2008; Williams, et al., 2007), and many studies have failed to adjust for these confounding factors in the relationship between HCV infection and depression. We conducted this study to examine the associations between HCV, somatic symptoms, and depression in a large cohort of HIV-infected patients in clinical care. We hypothesized that HCV would be associated with increased depression symptoms even after accounting for substance use and when measurement of depression did not include somatic symptoms that can be associated with both depression and HCV such as fatigue.
METHODS
Study setting
This cross-sectional study was conducted on a convenience sample of patients from the University of Washington (UW) HIV Cohort(H. M. Crane, et al., 2007; Kitahata, et al., 2003). This study received Institutional Review Board approval.
Study participants
HIV-infected patients over 18 years of age who attended the clinic for a routine appointment between 10/15/2005 and 5/11/2009 were eligible for the study. Patients receiving interferon were excluded given the well-known effect of interferon on depressive symptoms.
Data sources
As previously described(H. M. Crane, et al., 2007), patients used touch-screen tablet PCs to complete assessments of depression symptoms (PHQ-9 from the PRIME-MD)(Kroenke, Spitzer, & Williams, 2001; Spitzer, Kroenke, & Williams, 1999), substance use (Alcohol, Smoking, and Substance Involvement Screening Test [ASSIST])(Newcombe, Humeniuk, & Ali, 2005; 2002), alcohol risk (Alcohol Use Disorders Identification Test consumption questions [AUDIT-C])(Bradley, et al., 2003; Bush, Kivlahan, McDonell, Fihn, & Bradley, 1998), health-related quality of life (EuroQOL 5-dimension questionnaire [EQ-5D])(J. A. Johnson & Coons, 1998; J. A. Johnson, Coons, Ergo, & Szava-Kovats, 1998; Wu, et al., 2002), and symptoms (HIV Symptom Index)(Justice, et al., 2001).
Clinical data were obtained from the UW HIV Information System (UWHIS), a comprehensive database based on the electronic health records of patients belonging to the UW HIV cohort. It includes clinical data from all outpatient and inpatient encounters including demographic, clinical, laboratory, medication, and socioeconomic information.
HCV
Patients with positive HCV antibody, RNA, or genotype tests were considered HCV co-infected.
Instrument Scoring
Standard PHQ-9 scores range from 0–27 and are categorized as: none (0–4 points), mild (5–9 points), moderate (10–14 points), moderately-severe (15–19) and severe (≥20 points) depressive symptom severity(Kroenke, et al., 2001). PHQ-9 standard scores have curvilinear measurement properties with respect to the latent trait of depression defined by all the items, meaning that a constant difference in score implies different amounts of depression symptom severity at different depression severity levels(P. K. Crane, et al., 2010). In this situation, using continuous standard total scores in regressions can lead to confusing and even biased findings(P. K. Crane, et al., 2008). We therefore generated scores using item response theory (IRT) as done previously(P. K. Crane, et al., 2010); we refer to these throughout as “depression severity scores”. IRT-based depression severity scores have linear scaling properties with respect to the latent trait of depression defined by all the items(Embretson & Reise, 2000). To improve comprehensibility we transformed depression severity scores by multiplying by 15 and adding 100; this results in rescaling scores analogous to the IQ metric. We plotted a scatterplot showing the relationships between standard PHQ-9 scores and IRT-based depression severity scores.
Previously, we found a notable differences in mean depression scores between African-Americans and whites due to item-level bias, referred to as differential item functioning (DIF)(P. K. Crane, et al., 2010). We therefore used demographic specific item parameters to generate scores accounting for DIF related to age, race (African-American vs. white), sex, and HIV transmission risk factor; for this analysis we omitted individuals who were neither African-American nor white.
Many prior studies used depression measures that include somatic depression symptoms such as fatigue and sleep disturbance that may be impacted by chronic illnesses such as HCV(Dwight, et al., 2000; Golub, et al., 2004; Hilsabeck, Hassanein, & Perry, 2005; Lang, et al., 2006; McDonald, Jayasuriya, Bindley, Gonsalvez, & Gluseska, 2002; Perkins, et al., 1995; Poynard, et al., 2002; P. S. Sullivan & Dworkin, 2003; Tsao, Dobalian, Moreau, & Dobalian, 2004). This raises the question of whether associations between HCV and depression may be due in part to somatic symptoms associated with HCV rather than depression itself. We thus conducted a sensitivity analysis using a reduced depression severity score in which we removed the fatigue, loss of appetite, and sleep disturbance items from the depression instrument. We used IRT to generate this reduced depression score.
The HIV Symptom Index is a measure of 20 symptom groups(Justice, et al., 2001). Symptom scores were calculated based on the number of symptoms patients indicated as bothersome (excluding those symptoms that bothered the patient only a little), with higher scores indicating greater symptom burden. For these analyses, the depression/sadness symptom item was excluded. We also examined symptoms individually, categorizing each symptom as bothersome or not.
There are several ways to score the ASSIST to measure substance use(Newcombe, et al., 2005; 2002). We were interested in any illicit drug use, current use defined as any illicit drug within the prior 3 months, and identifying individual substance categories (opiates, amphetamine, or cocaine/crack).
The AUDIT-C scores for alcohol use were calculated by summing the scores for each AUDIT-C question (0–4 points each)(Bush, et al., 1998). We used a score of ≥5 for men and ≥4 for women to define at-risk alcohol use(Gual, Segura, Contel, Heather, & Colom, 2002).
The EQ-5D is a 5-item measure of health-related quality of life (HRQL). The combination of responses categorizes patients into one of 243 unique possible health states. Each health state is assigned a preference-based index score using general population-based weights(Shaw, Johnson, & Coons, 2005).
Statistical Analyses
We performed bivariate analyses comparing study participant characteristics to the entire UW HIV cohort using χ2 tests for categorical variables and t-tests for continuous variables. We compared demographic and clinical characteristics by HCV status using χ2 tests. We performed bivariate analyses of associations with depression severity scores using t-tests. To prevent overestimation of the prevalence of symptoms and behaviors, missing symptom or behavior items were assumed to be absent. We used an imputed height based on age, sex, and race for 15 individuals in whom height was missing. We calculated body mass index (BMI) using the traditional Quetelet index(1998). Baseline BMI was categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2).
Key variables included depression severity scores, HCV infection, demographic characteristics (age, self-reported race/ethnicity, sex, and risk factor for HIV transmission), symptoms, and clinical characteristics (CD4+ cell count nadir, current CD4+ cell count, peak HIV-1 RNA level, current antiretroviral therapy (ART) use, current anti-depressant medication use, at-risk alcohol use, any or current illicit drug use, and BMI). We used multivariate linear regression to examine the relationship between depression severity scores and HCV infection accounting for demographic and clinical characteristics.
In addition to the main models, we conducted a number of sensitivity analyses. We hypothesized that the effect of HCV on depression severity scores may be mediated, in part, through somatic depression symptoms and therefore excluded them from the main models. However, somatic symptoms including those not typically considered part of the construct of depression were included as covariates in models for sensitivity analyses as comparisons of these results to the main models can help determine the extent to which any relationship between HCV and depression may be mediated by somatic symptoms. To further explore the potential mediating relationship of symptoms, we also conducted adjusted analyses of symptoms and depressive severity scores excluding HCV and we conducted multivariate logistic regression analyses of HCV and individual symptoms. We constructed depression severity models that adjusted for HRQL scores. We conducted sensitivity analyses using depression severity scores adjusted for item-level bias. We conducted sensitivity analyses using standard PHQ-9 depression severity scores as the outcome. We conducted analyses using standard PHQ-9 depression severity categories defined by standard scores as the outcome; these models employed ordinal logistic regression. Two-tailed p values of <0.05 were considered significant for statistical tests. Analyses were conducted using Stata 9.2(StataCorp).
RESULTS
During the study period, the assessment was completed by 764 eligible HIV-infected patients of whom 160 (21%) had HCV infection (see Table 1). One patient receiving pegylated interferon therapy was excluded from the study. Completion rates were high with minimal missing data. Missing data rates for each symptom item were all <3%, with the highest rate for the nausea item (missing for 19/764, 2.5%). There were 28 patients who had missing data for the AUDIT-C (3.7%), and 44 patients who had missing data for any of the items for the EuroQOL (5.6%). The median age was 45 years old, 87% were men, the median current CD4+ T cell count was 393 cells/mm3, and the mean BMI was 26.6 kg/m2 (SD 5.0). At the time of the assessment, 172 patients (23%) were receiving antidepressant medications. Demographic and clinical characteristics of study patients were similar to those of all patients receiving care at the clinic during the study period (data not shown).
Table 1.
Clinical and demographic characteristics of HIV-infected study patients by HCV status (N=764)
| Characteristic | HCV-uninfected N = 604 |
HCV-infected N = 160 |
p-value |
|---|---|---|---|
| N (%) | N (%) | ||
| Sex | |||
| Male | 531 (88) | 133 (83) | |
| Female | 73 (12) | 27 (17) | 0.1 |
| Age (years) | |||
| < 30 | 43 (7) | 5 (3) | |
| 30–39 | 141 (23) | 23 (14) | |
| 40–49 | 261 (43) | 80 (50) | |
| ≥50 | 159 (26) | 52 (33) | 0.01 |
| Race | |||
| White | 399 (66) | 95 (59) | |
| Black | 105 (17) | 37 (23) | |
| Hispanic | 55 (9) | 12 (8) | |
| Other/Unknown | 45 (8) | 16 (10) | 0.2 |
| HIV transmission risk factor | |||
| MSM | 379 (63) | 38 (24) | |
| IDU | 91 (15) | 100 (63) | |
| Heterosexual | 99 (16) | 17 (11) | |
| Other | 35 (6) | 5 (3) | < 0.001 |
| BMI (kg/m2) | |||
| < 18.5 | 10 (1.7) | 1 (0.6) | |
| 18.5–24.9 | 240 (39.7) | 78 (49) | |
| 25–29.9 | 229 (37.9) | 45 (28) | |
| ≥ 30 | 125 (20.7) | 36 (23) | 0.07 |
| CD4+ cell count current (cells/mm3) | |||
| 0–200 | 103 (17) | 32 (20) | |
| 201–350 | 139 (23) | 46 (29) | |
| >350 | 362 (60) | 82 (51) | 0.1 |
| CD4+ cell count nadir (cells/mm3) | |||
| 0–200 | 327 (54) | 106 (66) | |
| 201–350 | 173 (29) | 34 (21) | |
| >350 | 104 (17) | 20 (13) | 0.02 |
| HIV-1 RNA level peak* (copies/ml) | |||
| ≥100,000 | 209 (35) | 58 (36) | |
| 10,000–99,999 | 196 (32) | 58 (36) | |
| <10,000 | 198 (33) | 44 (28) | 0.4 |
| Currently on ART | |||
| Yes | 423 (70) | 109 (68) | |
| No | 181 (30) | 51 (32) | 0.6 |
| Current illicit drug use (past 3 months) | |||
| Yes | 121 (20) | 68 (42) | |
| No | 483 (80) | 92 (58) | 0.001 |
| Current illicit drug use by drug category | |||
| Cocaine | 68 (11) | 41 (26) | < 0.001 |
| Speed | 71 (12) | 39 (24) | < 0.001 |
| Opiates | 10 (2) | 14 (9) | < 0.001 |
| At-risk alcohol use | |||
| Yes | 104 (17) | 25 (16) | |
| No | 500 (83) | 135 (84) | 0.6 |
| PHQ-9 depression categories | |||
| None (0–4) | 245 (41) | 45 (28) | |
| Mild (5–9) | 166 (27) | 42 (26) | |
| Moderate (10–14) | 95 (16) | 31(19) | |
| Moderately-severe (15–19) | 56 (9) | 26 (16) | |
| Severe (20–27) | 42 (7) | 16 (10) | 0.009 |
Missing HIV-1 RNA level data for one patient, therefore N = 763 for this category.
ART: antiretroviral therapy; BMI: body mass index; IDU: injection drug user; MSM: men who have sex with men
HCV-infected patients were more likely to have lower nadir CD4+ cell counts (382 vs. 433 cells/mm3, p = 0.01), to have been injection drug users (p<0.001), and to report current illicit drug use within the past 3 months (p<0.001). HCV-infected patients were more likely to report ever use and current use of cocaine, amphetamines, or opiates compared with those without HCV (p values all <0.001). HCV-infected patients were also more often prescribed antidepressant medication (p=0.03). There were no significant differences between those with HCV infection and those without in regards to sex, age, race/ethnicity, at-risk alcohol use, BMI, current CD4+ count, and peak HIV viral load (Table 1).
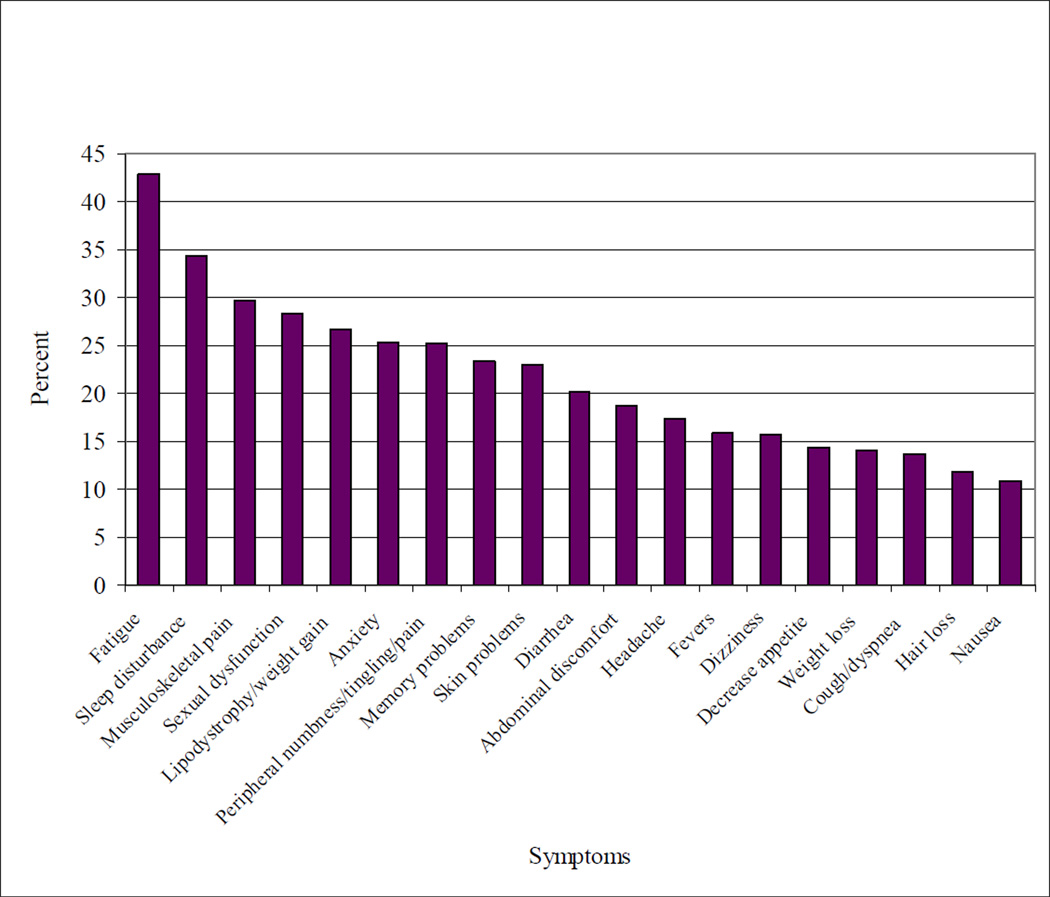
We found a high overall symptom burden, with patients reporting an average of 4.1 symptoms (SD 4.2). Fatigue (43%) and sleep disturbance (34%) were the most common symptoms endorsed (Figure 1). HCV-infected patients had a higher symptom burden on average than those without HCV, with a symptom score of 4.8 vs. 3.9 (p=0.02).
Figure 1.
Prevalence of moderate or severe symptoms among a population of HIV-infected patients (N = 764)
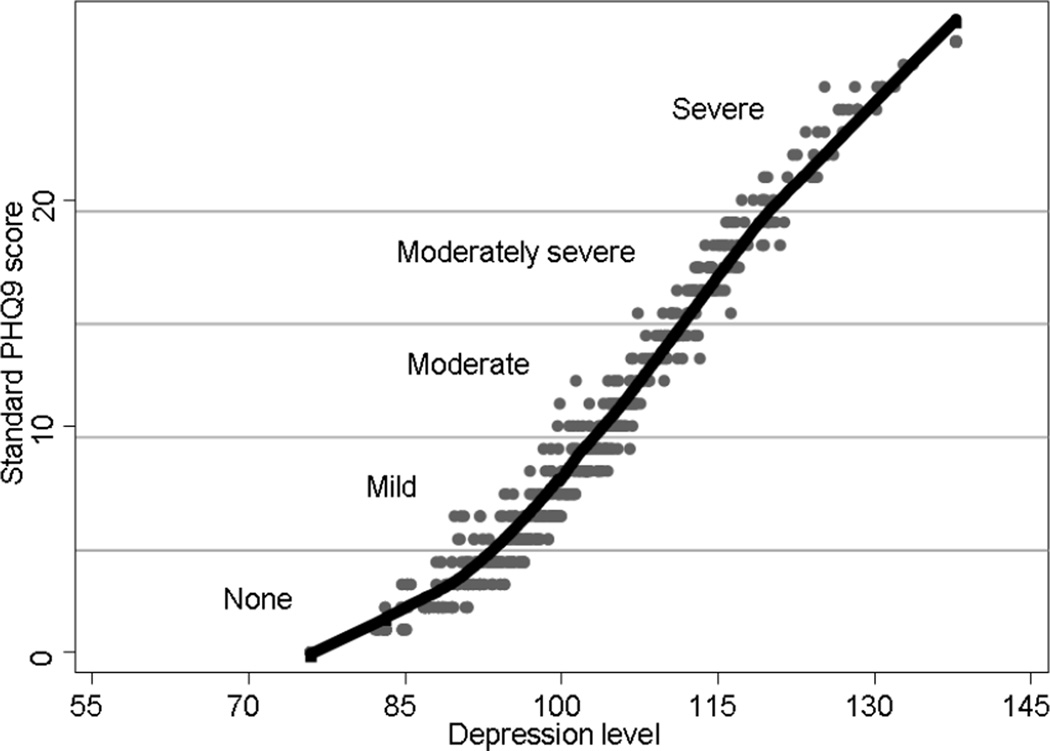
The curvilinear relationship between IRT depression severity scores and standard scores is shown in the scatterplot (Figure 2). Two hundred and ninety patients (38%) had no depression, 208 (27%) had mild, 126 (17%) had moderate, 82 (11%) had moderately-severe, and 58 (8%) had severe depression defined by standard scores(Kroenke, et al., 2001). Among HCV-infected patients, 73 (46%) were at least moderately depressed, compared to 193 (32%) of those without HCV (p = 0.001) (Table 1) and mean depression severity scores were higher in HCV-infected patients (102 vs. 98, p=0.002).
Figure 2.
Scatter plot and lowess curve of IRT depression scores with standard PHQ-9 scores*
* This graph shows the range of depression levels associated with each standard PHQ-9 score in the scatter plot with gray dots. Depression severity categories based on standard PHQ-9 scores are also shown. In IRT scoring, unlike standard scoring, items may receive unequal weight. Endorsing a particular frequency of a more severe depression symptom results in a higher depression severity score than endorsing the same frequency of a less severe depression symptom. For example, consider individuals with standard PHQ-9 scores of 15 (the bottom row of the “Moderately severe” category). IRT depression severity scores for these individuals range from 107 to 116, suggesting some variability in the severity of depression masked by equal weights applied to each item in the standard PHQ-9 scores. The range of IRT depression severity scores associated with each standard PHQ-9 scores varies from a single value (at the minimal and maximal values of 0 and 27 points) to a 10 point range (at standard scores of 7 points). The 10 point range represents 2/3 of a standard deviation.
The black lowess curve shows a curvilinear relationship between standard scores and the level of depression. For these reasons, we chose to use the IRT depression score rather than the standard PHQ-9 score in our regression analyses.
Multivariate linear regression analyses suggested that HCV infection was associated with higher depression severity scores when controlling for differences in age, sex, race, CD4+ count nadir, current CD4+ count, ART status, current illicit drug use, at-risk alcohol use, and BMI. Mean depression severity scores for HCV-infected patients were 3.4 points higher than for patients without HCV in adjusted analyses (Table 2). In addition, higher BMI, current illicit drug use, and at-risk alcohol use were associated with higher depression severity scores. The association between HCV and greater depression severity remained significant in models that also adjusted for antidepressant medication use (+2.9, p=0.02), and in models that also adjusted for any rather than current illicit drug use (+3.4, p=0.01), or both any and current illicit drug use (+2.9, p=0.03). Findings were similar in sensitivity analyses using standard PHQ-9 scores and in sensitivity analyses using IRT scores that accounted for DIF rather than naÔve IRT scores (data not shown). HCV infection was associated with more severe depression severity category in sensitivity analyses using ordinal logistic regression with standard PHQ-9 score-based depression symptom severity categories(Kroenke, et al., 2001)(Table 3). HCV infection remained associated with more severe depression in multivariate analyses using reduced depression severity scores constructed from the 6 non-somatic PHQ-9 items (+3.1, p=0.01).
Table 2.
Change in depression severity scores among HIV-infected patients in routine clinical care using multivariate linear regression (N=764)
| Change in depression severity score; 95% CI |
p value | |
|---|---|---|
| Hepatitis C infection | ||
| No | ref | |
| Yes | +3.4; 0.8–5.9 | 0.01 |
| Current CD4+ count (cells/mm3) | ||
| 0–200 | ref | |
| 201–350 | −4.6; −7.9–−1.4 | 0.005 |
| >350 | −6.1; 9.2–−3.0 | <0.001 |
| BMI (per kg/m2) | +0.2; 0.0–0.4 | 0.03 |
| Current illicit drug use | ||
| No | ref | |
| Yes | +4.4; 2.0–6.8 | <0.001 |
| At-risk alcohol use | ||
| No | ref | |
| Yes | +2.9; 0.1–5.6 | 0.04 |
Model adjusted for age, sex, race, CD4+ count nadir, current CD4+ count, ART status, current illicit drug use, at-risk alcohol consumption, and BMI.
BMI: body mass index
Table 3.
Adjusted odds ratios for more severe depression categories using multivariate ordinal logistic regression among HIV-infected patients in routine clinical care (N=764)
| Adherence | ||
|---|---|---|
| OR; 95% CI | p value | |
| Hepatitis C infection | ||
| No | 1 (ref) | |
| Yes | 1.6; 1.2–2.3 | 0.004 |
| Current CD4+ count (cells/mm3) | ||
| 0–200 | 1 (ref) | |
| 201–350 | 0.5; 0.4–0.8 | 0.005 |
| >350 | 0.4; 0.3–0.7 | <0.001 |
| BMI (per kg/m2) | 1.04; 1.02–1.08 | 0.001 |
| Current illicit drug use | ||
| No | 1 (ref) | |
| Yes | 1.6; 1.2–2.2 | 0.002 |
| At-risk alcohol use | ||
| No | 1 (ref) | |
| Yes | 1.5; 1.0–2.1 | 0.04 |
Model adjusted for age, sex, race, CD4+ count nadir, current CD4+ count, ART status, current illicit drug use, at-risk alcohol consumption, and BMI.
BMI: body mass index
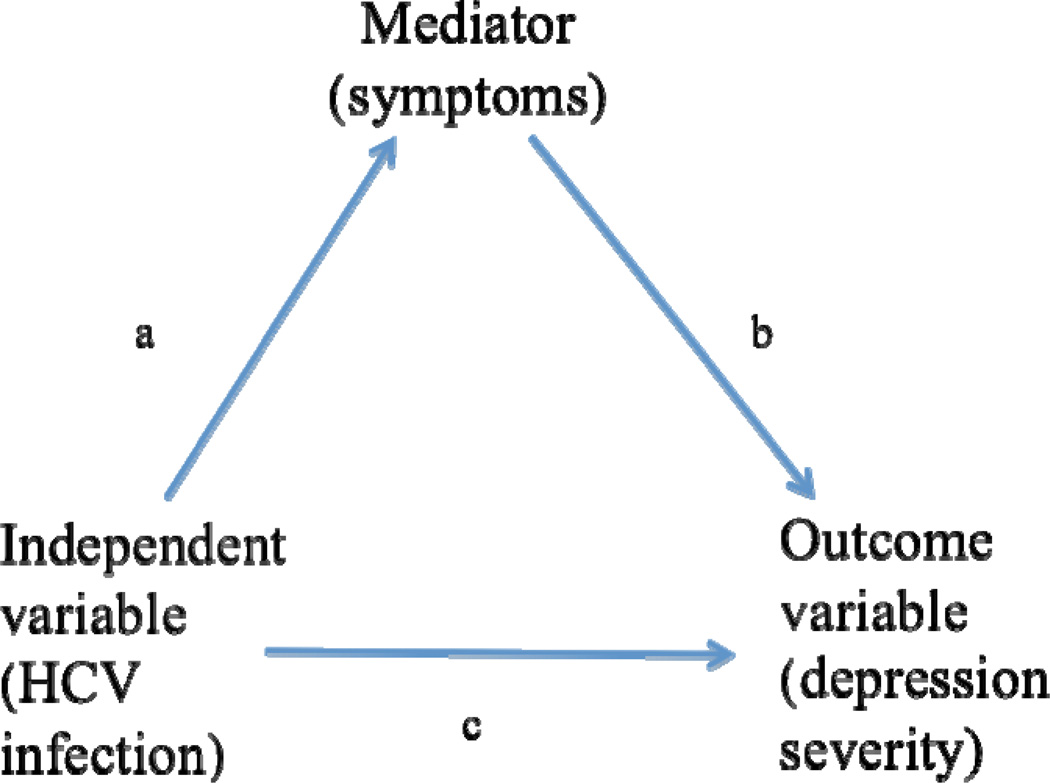
To examine whether somatic symptoms including symptoms not typically considered part of the depression construct could potentially be mediating the association between HCV and depression severity scores using a classic approach (Figure 3)(Baron & Kenny, 1986), we first demonstrated that HCV was associated with overall symptom burden scores (p=0.04) in adjusted analyses. Furthermore, HCV was also associated with a number of individual symptoms particularly memory problems, poor appetite, and nausea (p values 0.006–0.03). We then demonstrated in adjusted models without HCV, that higher overall symptom burden was associated with more severe depression severity scores (p<0.001). In models with individual symptoms rather than overall symptom burden, memory problems, poor appetite, fatigue, fevers, anxiety, sleep disturbance, sexual dysfunction, and weight loss were all associated with more severe depression symptom severity (p values<0.001–0.007). Finally, we examined the association between HCV and depression severity scores in adjusted models that included individual somatic symptoms or overall symptom burden scores. When somatic symptoms were included as individual covariates or as overall symptom burden, HCV infection was no longer significantly associated with higher depression severity scores (p values 0.09–0.1). Fatigue, subjective fevers, memory problems, anxiety, decreased appetite, sleep disturbance, sexual dysfunction, and weight loss were significantly associated with depression severity scores in adjusted models that included all symptoms. Depression severity scores constructed from the 6 non-somatic PHQ-9 items were associated with the same symptoms. Nausea, dizziness, and musculoskeletal pains were not associated with depression severity and were dropped from subsequent analyses.
Figure 3.
Mediating role of symptoms in the association between HCV infection and depression symptoms severity
*Note this figure is modified from (Baron & Kenny, 1986)
The mean EuroQOL score for the entire study cohort was 0.80 (SD 0.19). HCV-infected patients had worse HRQL, with mean EuroQOL score of 0.76 in HCV-infected patients vs. 0.81 in patients without HCV infection (p=0.01). After accounting for clinical and demographic characteristics (age, race, sex, recent illicit drug use, nadir and recent CD4+ counts and ART status), the association between HCV infection and HRQL was no longer significant (p=0.07).
DISCUSSION
We found a high prevalence and severity of depression among HIV-infected patients in routine care; particularly among those co-infected with HCV. The association between HCV and depression severity persisted when omitting somatic depression symptom items from the depression measure. However, the association was not seen in adjusted analyses that included somatic symptoms as covariates. Increased BMI, current illicit drug use, and at-risk alcohol use were also consistently associated with greater depression severity.
HCV infection is associated with depression in those without HIV infection(el-Serag, Kunik, Richardson, & Rabeneck, 2002; Gallegos-Orozco, et al., 2003; Goulding, O'Connell, & Murray, 2001; M. E. Johnson, Fisher, Fenaughty, & Theno, 1998; Lim, Cronkite, Goldstein, & Cheung, 2006; Obhrai, Hall, & Anand, 2001; Singh, Gayowski, Wagener, & Marino, 1997). However, there are conflicting data regarding the association between HCV and depression in HIV-infected patients(Backus, et al., 2005; Baillargeon, et al., 2008; Baum, et al., 2008; Grassi, et al., 2002; Libman, et al., 2006; Mrus, et al., 2006; Richardson, et al., 2005; von Giesen, et al., 2004). Our study supports an association between HCV and greater depression severity. Our conclusions are robust to several scoring methods including IRT estimates of depression severity from the whole scale and the non-somatic items, and depression categories and continuous scores defined using standard scoring. Furthermore, despite high prevalence rates of depression among patients with substance use issues, and a high prevalence of substance use among patients with HCV, we found that the association between HCV and greater depression severity persisted in numerous sensitivity analyses adjusting for current substance use, past substance use, or both.
Somatic symptoms such as fatigue and sleep disturbance may be due to depression as well as to chronic illnesses including HIV or HCV infection thereby obscuring examination of associations between HCV infection and depression(McDonald, et al., 2002; Perkins, et al., 1995; P. S. Sullivan & Dworkin, 2003; Tsao, et al., 2004). Studies have suggested that depression associated with HCV infection in HIV-infected patients may be attributable to somatic symptoms such as fatigue. For example, Braitstein and colleagues and Clifford and colleagues found that HIV-infected patients with HCV had higher CES-D somatic scores than those with HIV without HCV infection(Braitstein, et al., 2005; Clifford, Evans, Yang, & Gulick, 2005). In our analyses, including or excluding the somatic depression symptom items in IRT depression severity scores did not substantially impact the strength of association between HCV and depression. This suggests that the association between HCV and depression is not simply due to the patients meeting the clinical criteria for depression due to HCV-related somatic symptoms. Nevertheless, this finding does not exclude the possibility that HCV-related symptoms may be on a causal pathway leading to depression.
To further understand the potential role of somatic symptoms in possibly mediating the relationship between HCV and depression among HIV-infected patients, we examined the association between HCV and depression severity in models that included indicators for overall symptom burden or individual symptoms including those not considered part of the depression construct. In these models there was no longer an association between HCV and depression, suggesting that HCV-related somatic symptoms may mediate the relationship between HCV infection and depressive symptom severity. It may be that somatic symptoms associated with HCV cause depression. Alternatively, depressed patients may have a heightened awareness of symptoms due to their depression and the association of HCV and depression may in part be mediated by other factors. Only longitudinal studies can distinguish between these alternative explanations.
Potential limitations of our study include the reliance on self-reported data for some variables, and the use of a convenience sample of patients who completed the assessment, although our sample’s demographic and clinical characteristics were similar to the entire cohort. Additionally, the PHQ-9 questionnaire was not designed as a diagnostic tool although its validity has been evaluated previously, demonstrating good agreement with diagnoses made by mental health professionals(Spitzer, et al., 1999; Spitzer, et al., 1994). HCV co-infected patients could have been misclassified due to false negative HCV antibody tests or positive tests despite spontaneous HCV clearance. Negative HCV antibody test results among HCV co-infected patients occur, however it is uncommon(Forns & Costa, 2006), and spontaneous HCV clearance rates are lower among those with HIV than the general population(Grebely, et al., 2007). Furthermore, 139 of the 160 patients had a positive HCV RNA test. The cross-sectional nature of the study limits our ability to infer causal relationships in the association between HCV and depression. In addition, patients were recruited from a single large HIV clinic, so our findings may not be generalizable to all HIV-infected patients, especially those not in care.
Strengths of our study include the relatively large and diverse study population and standardized depression assessment. In addition, comprehensive clinical data facilitated adjustment for key potential confounding factors such as current and past illicit drug use, at-risk alcohol use, CD4+ cell count nadir, and current ART use. Furthermore, unlike some previous studies(12, 22, 46, 63), we avoided possible selection bias related to recruitment of patients from treatment trials, which tend to exclude patients with ongoing drug use or mental illness, or include patients with only more severe HCV disease.
In summary, we found high rates of depression in HIV-infected patients in routine care, particularly among those with HCV infection. The association between HCV and greater depression severity persisted even after taking into account associations between past or current substance use and depression severity. These findings suggest that there is an independent association between HCV and depression severity above and beyond any associations due to substance use. The association between HCV and depression persisted even when the depression measure excluded somatic items suggesting this association is not due to a mis-attribution of HCV-related somatic complaints toward the diagnosis of depression. Providers should be alert to the high rates of depression among HIV-infected patients in routine clinical care, particularly among those with HCV. Further longitudinal studies are needed to determine the casual role of HCV and HCV-related somatic symptoms in the development of depression.
ACKNOWLEDGEMENTS
We wish to thank the patients and providers of the University of Washington Madison HIV clinic. This work was supported by grants from the Mentored Patient-Oriented Research Career Development Award NIAID Grant (AI-60464), the NIH NIMH RO1 grant (RO1MH084759), the CFAR-Network of Integrated Clinical Systems (CNICS) (NIH R24 AI067039), and the University of Washington Center for AIDS Research NIAID Grant (AI27757).
ABBREVIATIONS
- HCV
Hepatitis C virus
- ART
antiretroviral therapy
- UW
University of Washington
- UWHIS
UW HIV Information System
- PHQ
patient health questionnaire
- PRIME-MD
Primary Care Evaluation of Mental Disorders
- IRT
item response theory
- HRQL
health-related quality of life
Footnotes
Data from this study was presented in part at the International Workshop on HIV Observational Databases, Sitges, Spain, March 2010.
The enclosed article has not been published and is not under consideration for publication elsewhere.
REFERENCES
- Backus LI, Boothroyd D, Deyton LR. HIV hepatitis C and HIV/hepatitis C virus co-infection in vulnerable populations. AIDS. 2005;19(Suppl 3):S13–S19. doi: 10.1097/01.aids.0000192065.09281.01. [DOI] [PubMed] [Google Scholar]
- Baillargeon JG, Paar DP, Wu H, Giordano TP, Murray O, Raimer BG, et al. Psychiatric disorders, HIV infection and HIV/hepatitis co-infection in the correctional setting. AIDS Care. 2008;20(1):124–129. doi: 10.1080/09540120701426532. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baum MK, Jayaweera DT, Duan R, Sales S, Lai S, Rafie C, et al. Quality of life, symptomatology and healthcare utilization in HIV/HCV co-infected drug users in Miami. J Addict Dis. 2008;27(2):37–48. doi: 10.1300/J069v27n02_05. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, et al. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163(7):821–829. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- Braitstein P, Montessori V, Chan K, Montaner JS, Schechter MT, O'Shaughnessy MV, et al. Quality of life, depression and fatigue among persons co-infected with HIV and hepatitis C: outcomes from a population-based cohort. AIDS Care. 2005;17(4):505–515. doi: 10.1080/09540120412331291733. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Buti M, Wong J, Casado MA, Esteban R. Quality of life and cost-effectiveness of anti-HCV therapy in HIV-infected patients. J Hepatol. 2006;44(1 Suppl):S60–S64. doi: 10.1016/j.jhep.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Evans SR, Yang Y, Gulick RM. The neuropsychological and neurological impact of hepatitis C virus co-infection in HIV-infected subjects. AIDS. 2005;19(Suppl 3):S64–S71. doi: 10.1097/01.aids.0000192072.80572.43. [DOI] [PubMed] [Google Scholar]
- Crane HM, Lober W, Webster E, Harrington RD, Crane PK, Davis TE, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Curr HIV Res. 2007;5(1):109–118. doi: 10.2174/157016207779316369. [DOI] [PubMed] [Google Scholar]
- Crane PK, Gibbons LE, Willig JH, Mugavero MJ, Lawrence ST, Schumacher JE, et al. Measuring depression levels in HIV-infected patients as part of routine clinical care using the nine-item Patient Health Questionnaire (PHQ-9) AIDS Care. 2010;22(7):874–885. doi: 10.1080/09540120903483034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Narasimhalu K, Gibbons LE, Mungas DM, Haneuse S, Larson EB, et al. Item response theory facilitated cocalibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol. 2008;61(10):1018–1027. e1019. doi: 10.1016/j.jclinepi.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwight MM, Kowdley KV, Russo JE, Ciechanowski PS, Larson AM, Katon WJ. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311–317. doi: 10.1016/s0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- el-Serag HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123(2):476–482. doi: 10.1053/gast.2002.34750. [DOI] [PubMed] [Google Scholar]
- Embretson S, Reise S. Item response theory for psychologists. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- Fleming CA, Christiansen D, Nunes D, Heeren T, Thornton D, Horsburgh CR, Jr, et al. Health-related quality of life of patients with HIV disease: impact of hepatitis C coinfection. Clin Infect Dis. 2004;38(4):572–578. doi: 10.1086/381263. [DOI] [PubMed] [Google Scholar]
- Forns X, Costa J. HCV virological assessment. J Hepatol. 2006;44(1 Suppl):S35–S39. doi: 10.1016/j.jhep.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Gallegos-Orozco JF, Fuentes AP, Gerardo Argueta J, Perez-Pruna C, Hinojosa-Becerril C, Sixtos-Alonso MS, et al. Health-related quality of life and depression in patients with chronic hepatitis C. Arch Med Res. 2003;34(2):124–129. doi: 10.1016/s0188-4409(03)00003-1. [DOI] [PubMed] [Google Scholar]
- Golub ET, Latka M, Hagan H, Havens JR, Hudson SM, Kapadia F, et al. Screening for depressive symptoms among HCV-infected injection drug users: examination of the utility of the CES-D and the Beck Depression Inventory. J Urban Health. 2004;81(2):278–290. doi: 10.1093/jurban/jth114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding C, O'Connell P, Murray FE. Prevalence of fibromyalgia, anxiety and depression in chronic hepatitis C virus infection: relationship to RT-PCR status and mode of acquisition. Eur J Gastroenterol Hepatol. 2001;13(5):507–511. doi: 10.1097/00042737-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Grassi L, Satriano J, Serra A, Biancosino B, Zotos S, Sighinolfi L, et al. Emotional stress, psychosocial variables and coping associated with hepatitis C virus and human immunodeficiency virus infections in intravenous drug users. Psychother Psychosom. 2002;71(6):342–349. doi: 10.1159/000065993. [DOI] [PubMed] [Google Scholar]
- Grebely J, Raffa JD, Lai C, Krajden M, Conway B, Tyndall MW. Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can J Gastroenterol. 2007;21(7):447–451. doi: 10.1155/2007/796325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gual A, Segura L, Contel M, Heather N, Colom J. AUDIT-3 and AUDIT-4: effectiveness of two short forms of the alcohol use disorders identification test. Alcohol Alcohol. 2002;37(6):591–596. doi: 10.1093/alcalc/37.6.591. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Hassanein TI, Perry W. Biopsychosocial predictors of fatigue in chronic hepatitis C. J Psychosom Res. 2005;58(2):173–178. doi: 10.1016/j.jpsychores.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Coons SJ. Comparison of the EQ-5D and SF-12 in an adult US sample. Qual Life Res. 1998;7(2):155–166. doi: 10.1023/a:1008809610703. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Coons SJ, Ergo A, Szava-Kovats G. Valuation of EuroQOL (EQ-5D) health states in an adult US sample. Pharmacoeconomics. 1998;13(4):421–433. doi: 10.2165/00019053-199813040-00005. [DOI] [PubMed] [Google Scholar]
- Johnson ME, Fisher DG, Fenaughty A, Theno SA. Hepatitis C virus and depression in drug users. Am J Gastroenterol. 1998;93(5):785–789. doi: 10.1111/j.1572-0241.1998.225_a.x. [DOI] [PubMed] [Google Scholar]
- Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl 1):S77–S90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Kanwal F, Gralnek IM, Hays RD, Dulai GS, Spiegel BM, Bozzette S, et al. Impact of chronic viral hepatitis on health-related quality of life in HIV: results from a nationally representative sample. Am J Gastroenterol. 2005;100(9):1984–1994. doi: 10.1111/j.1572-0241.2005.41962.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kim JH, Psevdos G, Suh J, Sharp VL. Co-infection of hepatitis B and hepatitis C virus in human immunodeficiency virus-infected patients in New York City, United States. World J Gastroenterol. 2008;14(43):6689–6693. doi: 10.3748/wjg.14.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahata MM, Dillingham PW, Chaiyakunapruk N, Buskin SE, Jones JL, Harrington RD, et al. Electronic human immunodeficiency virus (HIV) clinical reminder system improves adherence to practice guidelines among the University of Washington HIV Study Cohort. Clin Infect Dis. 2003;36(6):803–811. doi: 10.1086/368085. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CA, Conrad S, Garrett L, Battistutta D, Cooksley WG, Dunne MP, et al. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. J Pain Symptom Manage. 2006;31(4):335–344. doi: 10.1016/j.jpainsymman.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Libman H, Saitz R, Nunes D, Cheng DM, Richardson JM, Vidaver J, et al. Hepatitis C infection is associated with depressive symptoms in HIV-infected adults with alcohol problems. Am J Gastroenterol. 2006;101(8):1804–1810. doi: 10.1111/j.1572-0241.2006.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JK, Cronkite R, Goldstein MK, Cheung RC. The impact of chronic hepatitis C and comorbid psychiatric illnesses on health-related quality of life. J Clin Gastroenterol. 2006;40(6):528–534. doi: 10.1097/00004836-200607000-00012. [DOI] [PubMed] [Google Scholar]
- McDonald J, Jayasuriya J, Bindley P, Gonsalvez C, Gluseska S. Fatigue and psychological disorders in chronic hepatitis C. J Gastroenterol Hepatol. 2002;17(2):171–176. doi: 10.1046/j.1440-1746.2002.02669.x. [DOI] [PubMed] [Google Scholar]
- Mrus JM, Sherman KE, Leonard AC, Sherman SN, Mandell KL, Tsevat J. Health values of patients coinfected with HIV/hepatitis C: are two viruses worse than one? Med Care. 2006;44(2):158–166. doi: 10.1097/01.mlr.0000197027.06808.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity In Adults: The Evidence Report. 1998. Appendix 6. Body Mass Index: How To Measure Obesity; pp. 139–140. NIH Publication #98-4083. [PubMed] [Google Scholar]
- Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24(3):217–226. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- Obhrai J, Hall Y, Anand BS. Assessment of fatigue and psychologic disturbances in patients with hepatitis C virus infection. J Clin Gastroenterol. 2001;32(5):413–417. doi: 10.1097/00004836-200105000-00011. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Leserman J, Stern RA, Baum SF, Liao D, Golden RN, et al. Somatic symptoms and HIV infection: relationship to depressive symptoms and indicators of HIV disease. Am J Psychiatry. 1995;152(12):1776–1781. doi: 10.1176/ajp.152.12.1776. [DOI] [PubMed] [Google Scholar]
- Poynard T, Cacoub P, Ratziu V, Myers RP, Dezailles MH, Mercadier A, et al. Fatigue in patients with chronic hepatitis C. J Viral Hepat. 2002;9(4):295–303. doi: 10.1046/j.1365-2893.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- Richardson JL, Nowicki M, Danley K, Martin EM, Cohen MH, Gonzalez R, et al. Neuropsychological functioning in a cohort of HIV- and hepatitis C virus-infected women. AIDS. 2005;19(15):1659–1667. doi: 10.1097/01.aids.0000186824.53359.62. [DOI] [PubMed] [Google Scholar]
- Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62(6):957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34(6):831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- Singh N, Gayowski T, Wagener MM, Marino IR. Vulnerability to psychologic distress and depression in patients with end-stage liver disease due to hepatitis C virus. Clin Transplant. 1997;11(5 Pt 1):406–411. [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- Staples CT, Jr, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta VA (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29(1):150–154. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- Starace F, Ammassari A, Trotta MP, Murri R, De Longis P, Izzo C, et al. Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S136–S139. doi: 10.1097/00126334-200212153-00010. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software (Version 9.2) College Station, Texas: StataCorp.; [Google Scholar]
- Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288(2):199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Saitz R, Cheng DM, Libman H, Nunes D, Samet JH. The impact of alcohol use on depressive symptoms in human immunodeficiency virus-infected patients. Addiction. 2008;103(9):1461–1467. doi: 10.1111/j.1360-0443.2008.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Dworkin MS. Prevalence and correlates of fatigue among persons with HIV infection. J Pain Symptom Manage. 2003;25(4):329–333. doi: 10.1016/s0885-3924(02)00676-0. [DOI] [PubMed] [Google Scholar]
- Thein H, Maruff P, Krahn M, Kaldor J, Koorey D, Brew B, et al. Cognitive function, mood and health-related quality of life in hepatitis C virus (HCV)-monoinfected and HIV/HCV-coinfected individuals commencing HCV treatment. HIV Med. 2007;8(3):192–202. doi: 10.1111/j.1468-1293.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- Tsao JC, Dobalian A, Moreau C, Dobalian K. Stability of anxiety and depression in a national sample of adults with human immunodeficiency virus. J Nerv Ment Dis. 2004;192(2):111–118. doi: 10.1097/01.nmd.0000110282.61088.cc. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Heintges T, Abbasi-Boroudjeni N, Kucukkoylu S, Koller H, Haslinger BA, et al. Psychomotor slowing in hepatitis C and HIV infection. J Acquir Immune Defic Syndr. 2004;35(2):131–137. doi: 10.1097/00126334-200402010-00005. [DOI] [PubMed] [Google Scholar]
- WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- Williams DR, Gonzalez HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64(3):305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- Wu AW, Jacobson KL, Frick KD, Clark R, Revicki DA, Freedberg KA, et al. Validity and responsiveness of the euroqol as a measure of health-related quality of life in people enrolled in an AIDS clinical trial. Qual Life Res. 2002;11(3):273–282. doi: 10.1023/a:1015240103565. [DOI] [PubMed] [Google Scholar]