Abstract
Purpose
The treatment of symptomatic Schwannoma is surgical excision. However, in the case of major peripheral nerves with motor function, there are concerns including neurological complications following surgery. This study was designed to evaluate the surgical outcome of Schwannomas originating from major peripheral nerves of the lower limb. Additionally, we sought to find out the predictable factors for permanent neurological deficits.
Methods
Between 2004 and 2008, 30 consecutive Schwannomas underwent simple excision or enucleation. Surgical outcomes after excision were evaluated with an emphasis on neurological deficits and recurrence. Neurological complications were classified as major or minor neurological deficits and evaluated immediately after surgery and at final follow-up. Risk factors for development of neurological deficits were identified.
Results
Twenty-three patients (23/30, 76.7 %) developed neurological deficits immediately after surgery. After a mean of 58.8 months (32–79 months), 19 patients (19/30, 63.3 %) showed no residual neurological deficits. Among the remaining 11 (11/30, 36.7 %), nine patients had tolerable symptoms and two patients had major neurological deficits including significant motor weakness and sensory impairments. Larger tumours tended to be at greater risk of neurological deficit after surgery. One recurrence of the tumour was seen two years after surgery. There were no cases of reoperation or malignant transformation
Conclusions
In the majority of cases, Schwannomas in the lower limb can be excised with acceptable risk for neurological deficits. However, meticulous dissection is required in large-sized Schwannomas because these tumours seem to have a higher frequency of fascicular injury during dissection.
Introduction
Schwannoma is a benign peripheral nerve sheath tumour which arises from Schwann cells which support the peripheral nerve fibres. Malignant transformation is known to be exceptionally rare [1, 2]. The lesion is usually solitary, but multiple tumours in a limb have been reported [3]. Schwannomas most commonly occur in the head and neck involving the brachial plexus and spinal nerves. The lower limbs are affected less often [4]. Schwannomas usually grow slowly and present as a painless swelling over several years without specific symptoms. The diagnosis of a Schwannoma in a lower limb is often delayed for several years because it is frequently misdiagnosed as a benign solitary mass such as a ganglion, fibroma or myxoma [5, 6].
A Schwannoma is well encapsulated and eventually displaces the fascicles of the nerve, whereas a neurofibroma envelops them. For this reason, it is generally believed that a Schwannoma can easily be enucleated from the nerve without producing a neurological deficit. However, even with meticulou s dissection, several Schwannomas may not be separated or enucleated and this increases the risk of transient or permanent neurological damage [7–9]. The threshold for iatrogenic injury during surgical dissection tends to be lower in the extremities than in the trunk. In particular, a major neurological deficit such as motor weakness is critical in the lower limb. This study was conceived to (1) evaluate surgical outcomes of histopathologically confirmed Schwannomas originating from major peripheral nerves of the lower limb with motor function, focusing on any temporary or permanent neurological deficits; and (2) determine predictable factors for permanent neurological deficits following surgical excision.
Materials and methods
Before entering the study, informed consent and approval of the Institutional Review Board was obtained. Between January 2004 and August 2008, surgical excision of a Schwannoma of the lower limb was consecutively performed on 41 patients (44 Schwannomas). We included only histopathologically confirmed Schwannomas of the major peripheral nerves of the lower limb which had motor function. Those that were derived from cutaneous nerves or unidentified branches were excluded. Intramuscular Schwannomas or those that originated beneath the ankle joint were also excluded. Patients who had Schwannomas in multiple nerve sites were not included because of the difficulty in interpreting their postoperative outcomes. Thus, 30 patients (30 Schwannomas) were included in this study. There were 17 men and 13 women, with a mean age of 52.6 years (27–80 years). The mean postoperative follow-up was 58.8 months (32–79 years). No patients were lost during follow-up and there were no cases of plexiform Schwannoma.
The location and distribution of these tumours are summarised in Table 1. The initial complaint was of a palpable mass in 16, an incidentally detected mass in one, pain in two, paraesthesia in nine and hypoaesthesia in two cases. The mean interval between the onset of symptoms and excision was 41.1 months (2–360 months). Preoperative symptoms and signs are summarised in Table 2.
Table 1.
Location and nerves involved of 30 schwannomas
| Locations | Number | Nerves involved | Number |
|---|---|---|---|
| Buttock | 5 | Sciatic | 8 |
| Groin | 2 | Femoral | 3 |
| Thigh | 4 | Common peroneal | 9 |
| Popliteal fossa | 9 | Deep peroneal | 1 |
| Lower leg | 10 | Tibial | 9 |
Table 2.
Preoperative symptoms or signs of 30 Schwannomas
| Preoperative symptoms or signs | Number |
|---|---|
| Palpable mass | 27 (90 %) |
| Incidentally detected mass | 1 (3.3 %) |
| Rapid increase in size | 2 (6.7 %) |
| Motor weakness | 0 (0 %) |
| Spontaneous pain | 5 (16.7 %) |
| Paraesthesia | 15 (50 %) |
| Hypaesthesia | 3 (10 %) |
| Local tenderness | 14 (46.7 %) |
| Tinel’s sign | 22 (73.3 %) |
At the initial presentation, a peripheral nerve sheath tumour was diagnosed clinically when a mass in the line of a nerve was accompanied by a positive Tinel’s sign or sensory disturbance in the distribution of that nerve. Magnetic resonance imaging (MRI) was performed in 24 patients and an ultrasound in 14 patients. MRI correctly diagnosed a Schwannoma in all cases, while ultrasound suggested a ganglion or neurofibroma on four occasions. Although we did not perform a biopsy routinely because of the risk of iatrogenic injury, ultrasound-guided needle biopsy was carefully done in nine patients. In seven of nine patients, a fine-needle biopsy was performed in other departments of our hospital. Only two patients, who had vague symptoms or signs, underwent biopsy in the orthopaedic department. A diagnosis was confirmed in only five patients and was inconclusive in the rest. Four patients had a previous history of unsuccessful excision of the tumour at other hospitals.
Surgical technique
All operations were performed by a single surgeon under loupe magnification using a microsurgical technique. However, if there was an especially high possibility of fascicle injury or need for neural repair, we used a microscope. After a linear incision was centred over a tumour, the tumour and the nerve around it were exposed. The tumour was usually enveloped by a true capsule which consists of the perineurium of the nerve bundle of origin surrounded by a condensation of the deepest layers of the epineurium. The capsule was often covered by tortuous blood vessels. We carefully made a longitudinal incision in the epineurium and the uninvolved nerve fibres that splayed around the tumour were dissected and retracted extracapsularly. The onionskin-like epineurial tissue layers were meticulously peeled out until the shiny surface of the tumour was exposed. Gentle dissection along the plane of the tumour capsule from the epineurial layers usually allowed the tumour to be shelled out as a whole without disturbing the nerve fascicles. If not, the tumour was removed after first reducing its size. However, small fascicles entering either the capsule or the substance of the tumour were sometimes found as we approached the proximal and distal poles. We tried to isolate these fascicles to minimise damage to the nerve, but in some instances, it was impossible to divide the tumour clearly from the original nervous tissue. In that case, we tried not to damage the fascicles even though there might be the risk of incomplete removal. The mean maximum diameter of the tumours was 4.2 cm (1.2–20 cm)
Evaluation of clinical outcome
Clinical follow-up started one week postoperatively and continued at one, three and six months and then every subsequent year after surgery. At each follow-up, clinical outcomes were assessed for sensory disturbances such as hypoaesthesia, paraesthesia or neuropathic pain, and muscle power/weakness was tested according to the Medical Research Council (MRC) muscle strength grading system [10] by an independent investigator (JHL). Also, these values were converted into sensory and motor evaluation scales which were expressed as scores with a reference value of 100 points for normal contralateral lower limb function.
Immediately after surgery and at final follow-up, the neurological status of each patient was classified as a major or a minor deficit [11]. Major neurological deficits included marked hypoaesthesia or paraesthesia, motor weakness of grade 3 or less and neuropathic pain enough to lead to functional disability which limited the ability to perform activities of daily living. Minor deficits indicated tolerable symptoms which did not affect daily living—mild hypoaesthesia or paraesthesia—and mild motor weakness more than grade 4. Intraoperative findings focused on whether or not surgical enucleation was possible without nerve injury. Recurrence of the tumour, re-operation and malignant transformation were also examined.
Statistical analysis
Univariate analysis was carried out to identify predictable factors for permanent neurological deficits. Clinical and surgical variables including age, sex, location and nerve of origin as well as preoperative symptoms and intraoperative findings were compared between patients with neurological deficits and those who had no deficits at final follow-up. The two-sample t test or Mann–Whitney test were used for quantitative factors and Fisher’s exact test for categorical factors. In order to identify independent predictors of the neurological deficit at final follow-up and to correct for compounding factors, partial Spearman’s correlation coefficient analysis was performed. In addition, correlations between the permanent neurological deficit and failure of surgical enucleation and between the permanent neurological deficit and presence of axonal injury were analysed using Spearman’s correlation test. Statistical significance was set at a p value smaller than 0.05.
Results
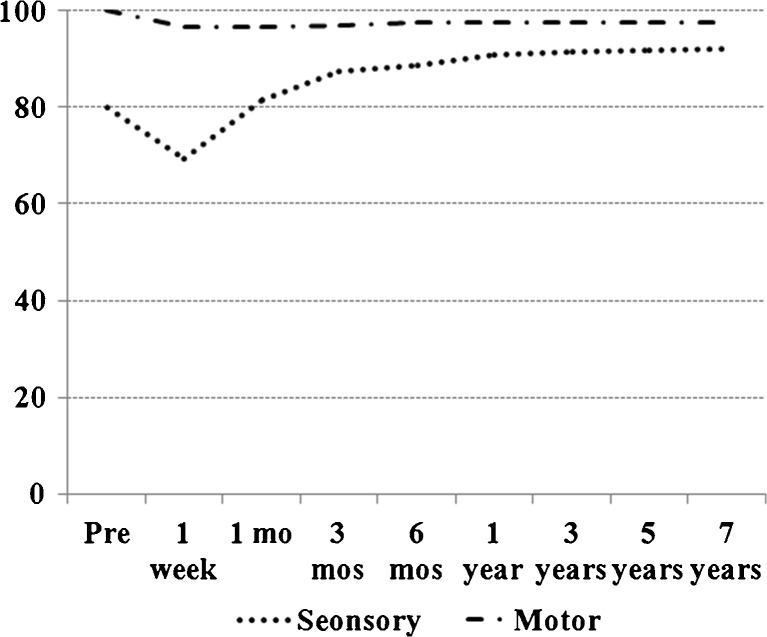
Mean sensory and motor evaluation scales of all 30 patients at postoperative week one then one, three and six months, one year and final follow-up are shown to represent overall neurological recovery with time (Fig. 1). Twenty-three patients (23/30, 76.7 %) developed neurological deficits immediately after surgery, while 19 patients (19/30, 63.3 %) showed no residual neurological deficit at final follow-up. Among the remaining 11 (11/30, 36.7 %) patients, nine had tolerable subjective symptoms and two patients had major neurological deficits including severe motor weakness and sensory impairments at final follow-up.
Fig. 1.
Overall neurological recovery with time of 30 Schwannomas
Among 23 patients who had neurological deficits immediately after surgery, seven patients complained of major neurological deficits. There were two with severe motor weakness, two with marked hypoaesthesia, two with marked paraesthesia and one with severe neuropathic pain. Of the 16 patients who had minor deficits, eight patients recovered fully within three months and two patients within one year. The remaining six patients recovered to some extent, but had persistent hypoaesthesia or paraesthesia which was tolerable and did not require further treatment.
Of the seven patients with major deficits immediately after surgery, five patients had improved gradually until final follow-up. One patient with severe motor weakness recovered gradually four months after surgery, with restoration of muscle power to grade 4 or more at the final follow-up. One patient with marked hypoaesthesia recovered to near normal without further treatment one year after the operation. One patient with marked hypoaesthesia and two patients with marked paraesthesia needed medication and nerve blocks. However, their symptoms gradually improved and only mild tingling persisted at final follow-up.
Two patients with major neurological deficits immediately after surgery still had marked complaints at final follow-up. Surgical enucleation failed in both patients and there may have been damage to the nerve tissue during the operation. A 41-year-old woman with a Schwannoma of the common peroneal nerve developed severe motor weakness equal to grade 3 and neuropathic pain after surgery. During her operation, nerve fascicles were found to enter into the mass of the tumour. She showed no evidence of recovery one year later and refused further management. In another case, a 27-year-old woman who underwent surgical excision of the tumour originating from the common peroneal nerve specified marked sensory deficits. Intraoperatively, two nerve fascicles were damaged during dissection and they were repaired primarily under a microscope. After surgery, she received continuous medication and nerve block therapy. Though her sensory impairments recovered gradually, mild paraesthesia and marked hypoaesthesia of the lower leg persisted.
Subjective satisfaction of the patients included “excellent” in 18 (18/30, 60 %), “good” in 8 (8/30, 26.6 %), “fair” in 2 (2/30, 6.7 %) and “poor” in 2 (2/30, 6.7 %). None of the above complications limited the daily activities of these patients except two who had severe motor weakness and marked hypoaesthesia. One recurrence of tumour was seen two years after surgery, but revealed no symptoms except a palpable mass. There were no cases of reoperation or malignant transformation. Four cases of revisional excision were performed successfully without complications or recurrence.
At final follow-up, the mean tumour size had been significantly larger in patients with neurological deficits than in those without (p < 0.01). Multivariate analysis demonstrated that tumour size was an independent predictor of postoperative neurological deficit following surgical excision of the Schwannoma (p < 0.01). Also, the mean diameter of the tumour was significantly greater in those with nerve axonal injury or failure of surgical enucleation (p = 0.0442 and p < 0.01, respectively). Neurological deficit was significantly correlated with the occurrence of nerve axonal injury and failure of surgical enucleation (p < 0.01 and p = 0.039, respectively).
Discussion
Benign nerve tumours include neurofibromas and Schwannomas. In contrast to a neurofibroma, in which complete excision of the mass inevitably leads to some damage to the parent nerve because the fascicles are embedded in the tumour, the Schwannoma, which arises from the neural sheath, is well encapsulated and surgical enucleation is generally believed to be routinely possible, producing little damage to the underlying nerve fascicles [3, 12, 13]. However, this is not completely true according to recent studies [14–16]. Even if the Schwannoma is carefully dissected from the involved nerve under magnification, neurological deficits sometimes occur. Oberle et al. reported immediate postoperative sensory deficits in six of 12 patients [8]. Donner et al. reported that 13 % of 85 Schwannomas in their series developed muscle weakness after the surgery [7]. Our study also showed that an immediate neurological deficit was seen in 76.7 % of patients, which suggests a high incidence of iatrogenic nerve injury during dissection. At final follow-up, 11 (36.7 %) of 30 patients had residual neurological deficits.
Many authors have reported their own individual surgical technique of excision for a Schwannoma [8, 15, 17, 18]. Some recommended extracapsular excision with good results [7, 14], but this is likely to damage the fascicles in the capsular layer during dissection. We believe that intracapsular enucleation is essential to minimise the risk of nerve injury. Much attention must be paid to meticulous dissection of the epineurial layer down to the shiny surface of the tumour, which allows the tumour to be shelled out safely while preserving the fascicles within the layers. In some cases, despite meticulous dissection, one or more fascicles were found to pass through the body of the tumour. In these cases, we tried to isolate tumour tissue from fascicles, but sometimes this was not successful. There have been some suggestions that resection of affected nerve fascicles did not induce neurological deficits because they usually had lost their function [7, 19]. However, the results of this study differ from these reports. In our study, postoperative neurological deficits were closely associated with failure of enucleation and intraoperative axonal injury. The transection of fascicles that run through the tumour is believed to be the major cause of postoperative neurological deficits. Sawada et al. hypothesised that the incision longitudinally in the sheath of the nerve may divide small numbers of fascicles held taut over the tumour mass [9]. Takase et al. mentioned that one of the main causes of new postoperative neurological deficits is damage to the fascicles along the tumour during dissection [20].
In our study, both MRI and ultrasound were used as diagnostic tools. Ultrasound, computed tomography and MRI have been used for preoperative assessment of Schwannomas [15]. Ultrasound defines the fascicular structure of nerves and allows the surgeon to define the site, size of the Schwannoma as well as its relationship to surrounding structures and helps to plan surgery [21]. Ultrasound is cheaper, does not expose the patient to radiation and is widely available compared to computed tomography and MRI.
It was found that the risk of developing neurological deficits was more likely to be high in patients with larger tumours. Some authors reported that tumour size may be a risk factor for neurological deficits [8, 22]. Park et al. observed that larger tumours tended to have more fascicles entering the tumour substance and were at greater risk of major neurological deficits after surgery [11]. On the basis of these findings, it is recommended that large-sized Schwannomas be managed meticulously with caution during surgery. Also, it would be expected that early surgical excision would have a better clinical outcome when a Schwannoma is detected in the major peripheral nerve of the lower limb. On the other hand, Oberle et al. reported that postoperative neurological deficits were associated with longer history [8]. In the study of Sawada et al., a positive preoperative Tinel’s sign showed a significant relationship with neurological complications [9]. However, there were no preoperative symptoms or signs which were predictable factors for final neurological outcomes except tumour size in our series. This may be due to the limited number of cases of the study.
We acknowledge that our study had some limitations. First, because of the rarity of this tumour, the number of cases was small and therefore, it was difficult to draw definitive conclusions. Second, this study was performed as a retrospective study of surgical excision of Schwannoma at different times. Some confounding factors from the study design may have affected the results. However, we believe that our results suggest important information to surgeons who intend to attempt surgical excision of Schwannomas derived from the major peripheral nerve. Identification of predictable factors related to neurological deficits may lead to strategies that can minimise surgical complications.
About one third of the patients had residual symptoms or signs at final follow-up. However, most patients had tolerable symptoms, i.e. mild hypoaesthesia or paraesthesia, none of which seemed to be troublesome or interfered with activities of daily living. Only two patients showed compromised clinical outcome including motor weakness and marked sensory impairment. Despite the high incidence of immediate neurological deficits, most showed improvement with time. Our final results were comparable to those of other studies [7, 14]. Kang et al. reported that of the 20 patients with excised Schwannoma, only one patient had persistent sensory loss [15]. Knight et al. mentioned that significant complications occurred in five of 198 patients available for follow-up evaluation [1].
In our study, we focused on Schwannomas arising from a major peripheral nerve having motor function. If motor function is damaged, significant functional disability including walking difficulty can occur. We believe that it is meaningful to investigate whether a Schwannoma arising from a major peripheral nerve of the lower limb shows a higher possibility of having neurological sequelae, compared to that arising from a muscular nerve or digital nerve. In addition, we sought to find independent risk factors of postoperative neurological deficits using univariate and multivariate analysis.
To conclude, a Schwannoma arising from a major peripheral nerve in the lower limb could be excised with an acceptable risk of nerve injury, although a transient neurological deficit occurred relatively often. However, new and/or permanent neurological deficits can develop in some patients following surgery. Therefore, all patients undergoing surgical excision of a Schwannoma arising from a major peripheral nerve of the lower limb must be informed about this complication. Meticulous attention to detail is required for large-sized Schwannomas because these tumours seem to have a higher risk of fascicular injury during dissection.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Knight DM, Birch R, Pringle J. Benign solitary Schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382–387. doi: 10.1302/0301-620X.89B3.18123. [DOI] [PubMed] [Google Scholar]
- 2.Nawabi DH, Sinisi M. Schwannoma of the posterior tibial nerve: the problem of delay in diagnosis. J Bone Joint Surg Br. 2007;89:814–816. doi: 10.1302/0301-620X.89B6.19077. [DOI] [PubMed] [Google Scholar]
- 3.Phalen GS. Neurilemmomas of the forearm and hand. Clin Orthop Relat Res. 1976;114:219–222. [PubMed] [Google Scholar]
- 4.Birch R, Bonney G, Wynn Parry CB (1998) The peripheral nervous system and neoplastic disease. In: Surgical disorders of the peripheral nerves. Churchill Livingstone, Edinburgh, pp 335–352
- 5.Ghaly RF. A posterior tibial nerve neurilemoma unrecognized for 10 years: case report. Neurosurgery. 2001;48:668–672. doi: 10.1097/00006123-200103000-00045. [DOI] [PubMed] [Google Scholar]
- 6.Rockwell GM, Thoma A, Salama S. Schwannoma of the hand and wrist. Plast Reconstr Surg. 2003;111:1227–1232. doi: 10.1097/01.PRS.0000046039.28526.1A. [DOI] [PubMed] [Google Scholar]
- 7.Donner TR, Voorhies RM, Kline DG. Neural sheath tumors of major nerves. J Neurosurg. 1994;81:362–373. doi: 10.3171/jns.1994.81.3.0362. [DOI] [PubMed] [Google Scholar]
- 8.Oberle J, Kahamba J, Richter HP. Peripheral nerve Schwannomas–an analysis of 16 patients. Acta Neurochir (Wien) 1997;139:949–953. doi: 10.1007/BF01411304. [DOI] [PubMed] [Google Scholar]
- 9.Sawada T, Sano M, Ogihara H, Omura T, Miura K, Nagano A. The relationship between pre-operative symptoms, operative findings and postoperative complications in Schwannomas. J Hand Surg Br. 2006;31:629–634. doi: 10.1016/j.jhsb.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 10.James MA. Use of the Medical Research Council muscle strength grading system in the upper extremity. J Hand Surg Am. 2007;32:154–156. doi: 10.1016/j.jhsa.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Park MJ, Seo KN, Kang HJ. Neurological deficit after surgical enucleation of Schwannomas of the upper limb. J Bone Joint Surg Br. 2009;91:1482–1486. doi: 10.2106/JBJS.H.00302. [DOI] [PubMed] [Google Scholar]
- 12.Kehoe NJ, Reid RP, Semple JC. Solitary benign peripheral-nerve tumours. Review of 32 years’ experience. J Bone Joint Surg Br. 1995;77:497–500. [PubMed] [Google Scholar]
- 13.Strickland JW, Steichen JB. Nerve tumors of the hand and forearm. J Hand Surg Am. 1977;2:285–291. doi: 10.1016/s0363-5023(77)80128-7. [DOI] [PubMed] [Google Scholar]
- 14.Artico M, Cervoni L, Wierzbicki V, D’Andrea V, Nucci F. Benign neural sheath tumours of major nerves: characteristics in 119 surgical cases. Acta Neurochir (Wien) 1997;139:1108–1116. doi: 10.1007/BF01410969. [DOI] [PubMed] [Google Scholar]
- 15.Kang HJ, Shin SJ, Kang ES. Schwannomas of the upper extremity. J Hand Surg Br. 2000;25:604–607. doi: 10.1054/jhsb.2000.0472. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Jung HG, Park YC, Kim HS. Results of neurilemoma treatment: a review of 78 cases. Orthopedics. 2001;24:977–980. doi: 10.3928/0147-7447-20011001-18. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246–255. doi: 10.3171/jns.2005.102.2.0246. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir O, Ozsoy MH, Kurt C, Coskunol E, Calli I. Schwannomas of the hand and wrist: long-term results and review of the literature. J Orthop Surg (Hong Kong) 2005;13:267–272. doi: 10.1177/230949900501300309. [DOI] [PubMed] [Google Scholar]
- 19.Yamane SMA, Kato H, Suenaga N, Iwasaki T. Neurilemmoma in the brachial plexus [in Japanese] J Jpn Soc Surg Hand. 2002;19:167–170. [Google Scholar]
- 20.Takase K, Yamamoto K, Imakiire A. Clinical pathology and therapeutic results of neurilemmoma in the upper extremity. J Orthop Surg (Hong Kong) 2004;12:222–225. doi: 10.1177/230949900401200216. [DOI] [PubMed] [Google Scholar]
- 21.Kuo YL, Yao WJ, Chiu HY. Role of sonography in the preoperative assessment of neurilemmoma. J Clin Ultrasound. 2005;33:87–89. doi: 10.1002/jcu.20085. [DOI] [PubMed] [Google Scholar]
- 22.Ogose A, Hotta T, Morita T, Otsuka H, Hirata Y. Multiple Schwannomas in the peripheral nerves. J Bone Joint Surg Br. 1998;80:657–661. doi: 10.1302/0301-620X.80B4.8532. [DOI] [PubMed] [Google Scholar]