Figure 1.
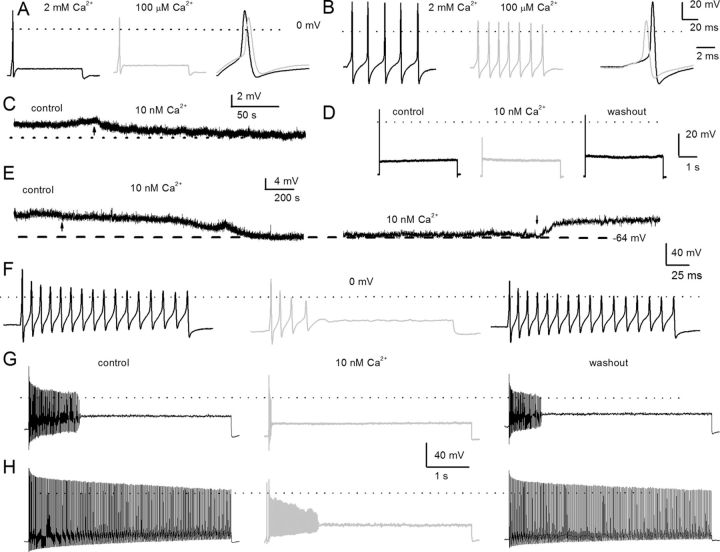
Effects of changes in external Ca2+ on action potentials in posthearing spiral ganglion neurons. A, We used the perforated-patch configuration to evoke electrical activity from apical, rapidly adapting SGNs in 2 mm extracellular Ca2+ (left, black) and after (middle, light gray) bath perfusion of extracellular solution containing ∼100 μm Ca2+. The magnitude and duration of the injected current were 0.2 nA and 100 ms, respectively. On the right panel, we superimposed evoked action potentials recorded in 2 mm Ca2+ and 100 μm Ca2+ solution. The peaks of the action potentials were 28 ± 3 mV in 2 mm Ca2+, and 10 ± 6 mV in 100 μm Ca2+ bath solutions (n = 8; p < 0.05), and the maximum right slopes were −99 ± 16 mV/ms in 2 mm Ca2+, and −78 ± 4 mV/ms in 100 μm Ca2+ bath solution (n = 8; p < 0.05). B, Evoked action potentials were elicited with a 0.05 nA current for ∼100 ms from a basal, slowly adapting, SGN in a bath solution containing 2 mm Ca2+ (left) and after (middle) a bath perfusion of ∼100 μm Ca2+solution. The right panel shows a comparison of action potential profiles in the two treatment conditions. The dotted lines show 0 mV levels. The peaks of the action potentials were 37.0 ± 7 mV in 2 mm Ca2+, and 16 ± 3 mV in 100 μm Ca2+ bath solutions (n = 9; p < 0.05), and the maximum right slopes were −149 ± 1 mV/ms in 2 mm Ca2+, and −83 ± 15 mV/ms in 100 μm Ca2+ bath solution (n = 9; p < 0.05). C, The characteristic rmp of a 3-month-old apical SGN. Reduction of external Ca2+ from 2 mm to ∼10 nm produced modest hyperpolarization of the rmp. The summary data show that the rmp for apical neurons was, for control, −58 ± 6 mV and, for 10 nm Ca2+, −61 ± 4 mV (n = 9; p = 0.12). The effect of reduced Ca2+ was reversible after washout with control solution. D, Injection of 0.2 nA current produced single spikes in apical SGNs. E, Exemplary current-clamp recordings from SGNs from the basal turn of the cochlea showed the rmp in control solutions and after application of 10 nm Ca2+ solution. The effects of 10 nm Ca2+ solution were reversible. Summary data from 15 basal SGNs show that the rmp in control solution was −55 ± 5 mV and in 10 nm Ca2+ solution was −64 ± 3 mV (n = 15; p < 0.05). F–H, The spike frequency of adult basal SGNs was heterogenous. Data from the same neuron after injection of 0.2 nA current for ∼200 ms (F) and ∼5 s (G), respectively. In 10 nm external Ca2+, the spike frequency plummeted by ∼5-fold compared with control (control, 17 ± 7 Hz; 10 nm Ca2+, 3 ± 2 Hz; n = 9; p < 0.05). Upon washout, the spike frequency was restored to 12 ± 7 Hz (n = 9; p = 0.08). H, In slowly adapting SGNs that fire unabatedly after injecting 0.2 nA for 5 s, the spike frequency was reduced by ∼4-fold after application of 10 nm Ca2+ solutions. From control to 10 nm Ca2+ solution, the spike frequency changed from 49 ± 10 Hz (control) to 12 ± 6 Hz (10 nm Ca2+) (n = 11; p < 0.05). The firing frequency after washout was 39 ± 14 Hz (n = 11; p = 0.11).