Fig. 5.
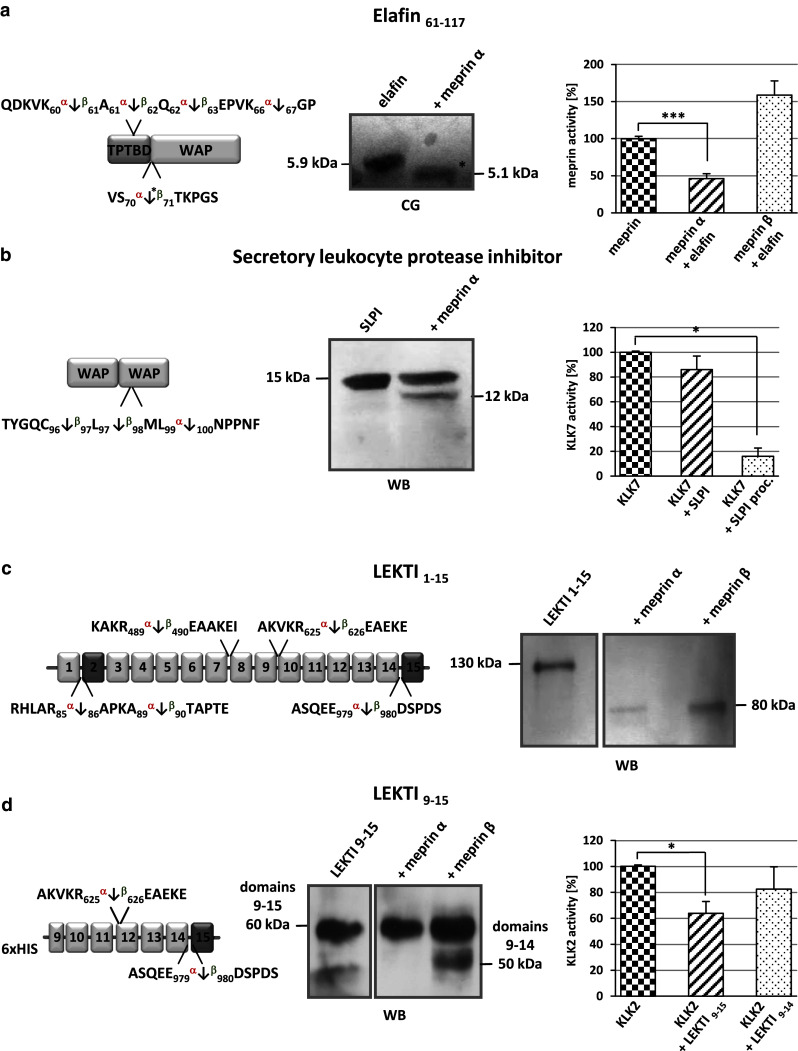
Inhibitors identified by TAILS as substrates for meprin α and β. a Elafin processed by meprin α was subjected to SDS-PAGE, transferred to a PVDF membrane and visualized by coomassie blue (CB) staining. The cleavage site identified by TAILS is indicated by the asterisk. The presence of elafin (5 × 10−5 M) leads to a significant reduction in meprin α activity measured by Mca-YVADAPK(Dnp)-NH2 cleavage. Significance was determined by the t test (***P < 0.001). b Cleavage of SLPI by meprin α was demonstrated by western blot (WB) analysis revealing a 12-kDa band. Incubation of KLK7 with meprin α-processed SLPI (SLPI proc.) resulted in increased inhibition compared to incubation with untreated SLPI. c, d Processed fragments of full-length LEKTI 1–15 and LEKTI 9–15 were detected by western blotting (WB) using a polyclonal LEKTI antibody. The full-length protein was cleaved by meprin α and β, both releasing an 80-kDa fragment. Specific processing of LEKTI 9–15 by meprin β resulted in a shift from 60 to 50 kDa, matching the TAILS data. Incubation of KLK2 with LEKTI 9–15 resulted in lower proteolytic activity than incubation with meprin β-cleaved LEKTI 9–14
