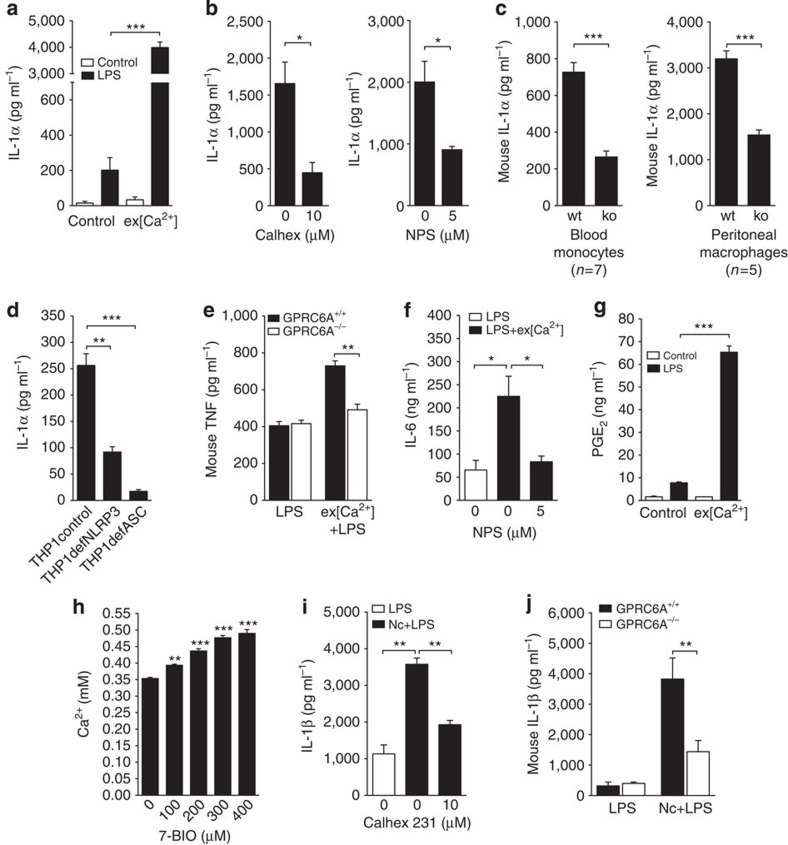
Figure 4. Extracellular calcium induces several proinflammatory cytokines and is released from necrotic cells.
(a) IL-1α release from unprimed (white bars) and LPS-primed (black bars) monocytes in response to stimulation with ex[Ca2+] (n=3). (b) Influence of the specific inhibitors Calhex231 and NPS2143 on IL-1α secretion of monocytes stimulated with increased ex[Ca2+]+LPS (n=3). (c) ex[Ca2+]-induced IL-1α secretion of CD11b+ mononuclear cells isolated from peripheral blood and of peritoneal macrophages from GPRC6A+/+ (wt) and GPRC6A−/− (ko) mice. (d) IL-1α secretion of THP-1 cells (control), NLRP3-deficient THP-1 cells (THP1defNLRP3) and ASC-deficient THP-1 cells (THP1defASC) stimulated with increased ex[Ca2+] concentration and LPS, after 16 h of culture (n=3). (e) Secretion of TNF from LPS-stimulated peritoneal macrophages or from macrophages stimulated with ex[Ca2+]+LPS from GPRC6A+/+ (wt) or GPRC6A−/− (ko) mice (n=3). (f) Influence of the specific inhibitor NPS2143 on IL-6 secretion of monocytes stimulated with increased ex[Ca2+]+LPS (n=3). (g) Prostaglandin E2 (PGE2) release from unprimed (white bars) and LPS-primed (black bars) monocytes in response to stimulation with ex[Ca2+] (n=3). (h) Influence of 7-BIO-induced monocyte necrosis on the Ca2+ concentration in the supernatant (n=3). (i) IL-1β release from LPS-primed CD14+ monocytes cultured alone (white bars) or co-cultured with necrotic autologous CD4+T cells (black bars) in the absence or presence of the specific inhibitor Calhex231 (n=3). (j) mIL-1β secretion of LPS-primed monocytes from GPRC6A+/+ (wt) and GPRC6A−/− (ko) mice cultured alone (LPS) or co-cultured with necrotic autologous CD4+ T cells (Nc+LPS, n=9). All bars show mean±s.e.m. Statistical analysis was performed using the two-tailed Student’s t-test. *P<0.05, **P<0.01 and ***P<0.001.